Introduction
Hypovitaminosis D, defined as vitamin D insufficiency, deficiency, or both, is a global health problem affecting all age groups and several at-risk groups in particular. Pregnant women are one of these high-risk groups, with a reported prevalence of hypovitaminosis D between 20% and 40%.1 Serum 25(OH)D concentration is the best indicator of vitamin D levels in the body, as it has a half-life of 15 days.2
A growing number of studies in the literature have assessed vitamin D deficiency during pregnancy and its association with maternal-fetal health. Several authors have performed systematic reviews, meta-analyses, and other studies demonstrating that low serum 25(OH)D levels in pregnant women are associated with obstetric and neonatal complications, such as increased risk of preeclampsia (PE), gestational diabetes mellitus (GDM), bacterial vaginosis, prematurity, and low birth weight.3-8 Children of women with hypovitaminosis D during pregnancy have been reported to be at increased risk for autoimmune diseases in childhood and adulthood, including diabetes mellitus type I, multiple sclerosis, allergies, and atopic diseases.9
Brazil is a tropical country with high sun exposure, but the climate varies widely throughout the country due to its geographic extension. In addition, the Brazilian population is characterized by ethnic diversity. Studies of the prevalence of hypovitaminosis D in the general Brazilian population are scarce, but some studies focusing on specific regions and groups have been published. These have shown a higher frequency of hypovitaminosis D in institutionalized elderly and postmenopausal women living in the city of São Paulo (latitude 23ºS).10,11 No Brazilian studies have evaluated serum vitamin D levels in pregnant women to date.
The present study aimed to investigate serum 25(OH)D levels in pregnant women in South Brazil and explore the impact of seasonality on those levels. Vitamin D levels are currently not routinely assessed in prenatal care in Brazil, and this study can help to inform whether it should be part of routine clinical care.
Materials and methods
This was a prospective cross-sectional study. Sample calculation was based on the number of births in the Curitiba metropolitan region in 2015, according to data from the Secretary of Health of the State of Paraná. The estimated sample size to obtain a 95% confidence level was 382 individuals. Serum vitamin D levels were tested at least four weeks after the beginning of the Summer or Winter season. All women were enrolled in prenatal outpatient clinics of public hospitals, specifically in Hospital de Clínicas (Clinics Hospital) of the Universidade do Paraná (Federal University of Paraná) and Hospital Universitário Evangélico (Evangelical University Hospital) in Curitiba, which are tertiary care hospitals.
Inclusion criteria comprised age between 18 and 40 years, Brazilian nationality with residence in the Curitiba metropolitan region, singleton pregnancy, and willingness to sign informed consent. Exclusion criteria included current use of vitamin D supplementation or chronic medications, such as anticonvulsants or glucocorticoids, and diseases other than those defining the high-risk group: preeclampsia (PE), gestational diabetes mellitus (GDM), or human immunodeficiency virus seropositivity (HIV+). Women in the low-risk group had no known conditions.
A questionnaire was developed by researchers and applied to participating women during an interview to collect sociodemographic, epidemiologic, and clinical data, including age, educational level, monthly household income, self-declared ethnicity, skin type according to Fitzpatrick´s 1988 classification, menarche, parity, date of the last menstrual period, gestational age according to ultrasonography, lifestyle habits (smoking, alcohol consumption, sedentary habits), weight at the first prenatal visit, height, body mass index (BMI), and current weight.12
Assessment of serum vitamin D levels
Venous blood samples were collected from all subjects in the morning. Samples were maintained at 2-8 °C and protected from light after collection and during transportation. Blood was centrifuged for 15 minutes at 3000 rpm for serum separation. All blood samples collected were analyzed on the same day of collection at the same laboratory using the microparticle chemiluminescence method with Architect i2000SR (Abbott, Illinois, USA).
Endocrine Society criteria were used to classify vitamin D status, with sufficiency being defined as vitamin D levels ≥30 ng/mL or 75 nmol/L, insufficiency as vitamin D levels between 20 and 30 ng/mL or 50 and 75 nmol/L, and deficiency as vitamin D levels below 20 ng/mL or 50 nmol/L.13
Statistical analysis
Data were collected in frequency and contingency tables using Microsoft Excel 2013®. Quantitative data were described as mean and standard deviation or median and minimum and maximum values. Qualitative data were described as frequencies and percentages. The Kolmogorov-Smirnov test was used to assess the normality of quantitative variables. T-test for independent samples was used for comparison of quantitative data between the two groups assessed. One-way analysis of variance model and the least significant difference test were used for multiple comparisons. Qualitative variables were compared through Chi-square or Fisher's exact test. A p-value ≤0.05 indicated statistical significance. Data were analyzed using Stata v.13.1 software (Texas, USA).
Ethics
This study was approved by the Research Ethics Committee of the Clinics Hospital of the Federal University of Paraná and of the Evangelical University Hospital of Curitiba. All subjects agreed to participate in the study and signed informed consent.
Results
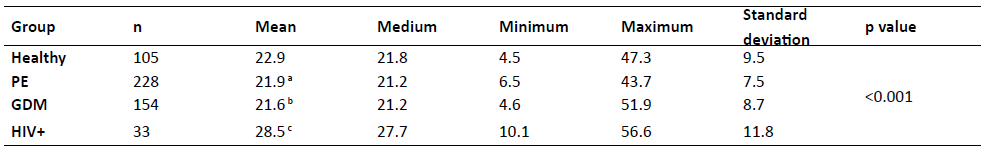
A total of 520 pregnant women were included in the study in 2016. Of these, 256 (49.2%) were assessed in the Summer (January through March) and 264 (50.8%) in the Winter (July through August; Table 1). Twenty percent of women (n=105) were included in the low-risk group, and the remaining 80% (n=415) in the high-risk group, with 228 women in this group presenting with PE, 154 with GDM, and 33 with HIV+.
Table 1 Clinical, epidemiological, and demographic data of pregnant Brazilian women

BMI, body mass index; LMP, last menstrual period; SD, standard deviation; US, ultrasound
The overall study population had a mean age of 29.5 ± 6.1 years, a mean age of menarche of 12.6 ± 1.6 years, a mean chronological gestational age of 32.0 ± 6.4 weeks, and a mean ultrasound gestational age of 32.1 ± 6.4 weeks. Nearly half of women had Fitzpatrick skin type II, and most were overweight at the beginning of gestation, with a mean BMI of 28.6 ± 6.7 kg/m2, a mean weight gain until the date of data collection of 8.2 ± 6.8 kg, and a mean BMI at that time of consultation of 30.3 ± 5.9 kg/m2. Sedentary lifestyle was common in this cohort (85.5%). Most women were nonsmokers and had no tobacco or alcohol consumption habits.
The mean serum 25(OH)D level of the study cohort was 22.5 ± 8.7 ng/mL, being significantly higher in Summer (mean 26.7 ± 7.8 ng/mL, range 8.8-56.6 ng/mL) compared to Winter (mean 18.3 ± 7.5 ng/mL, range 4.5-51.9 ng/mL; p <0.001) months. A total of 19.2% of women had vitamin D deficiency in the Summer compared to 67.4% in the Winter. Vitamin D insufficiency was observed in 50.8% of cases in the Summer and 23.9% in the Winter, and vitamin D sufficiency in 30.0% and 8.7%, respectively. All these differences were statistically significant (p <0.001; Table 2).
Table 2 Serum vitamin D levels in pregnant women during Summer and Winter
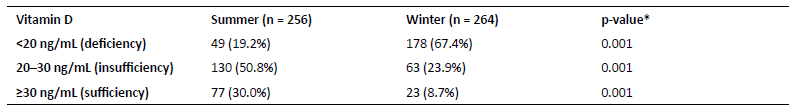
*t-test for independent samples
As shown in Table 3, age, education, ethnicity, skin type, and smoking were not significantly associated with serum vitamin D levels during Summer. Primiparous women had a significantly higher incidence of vitamin D deficiency than multiparous counterparts (p=0.026). Women with an initial BMI greater than 30 kg/m2 were significantly less likely to be vitamin D-deficient (p=0.014).
Table 3 Association between maternal characteristics and serum vitamin D levels during Summer in Brazil
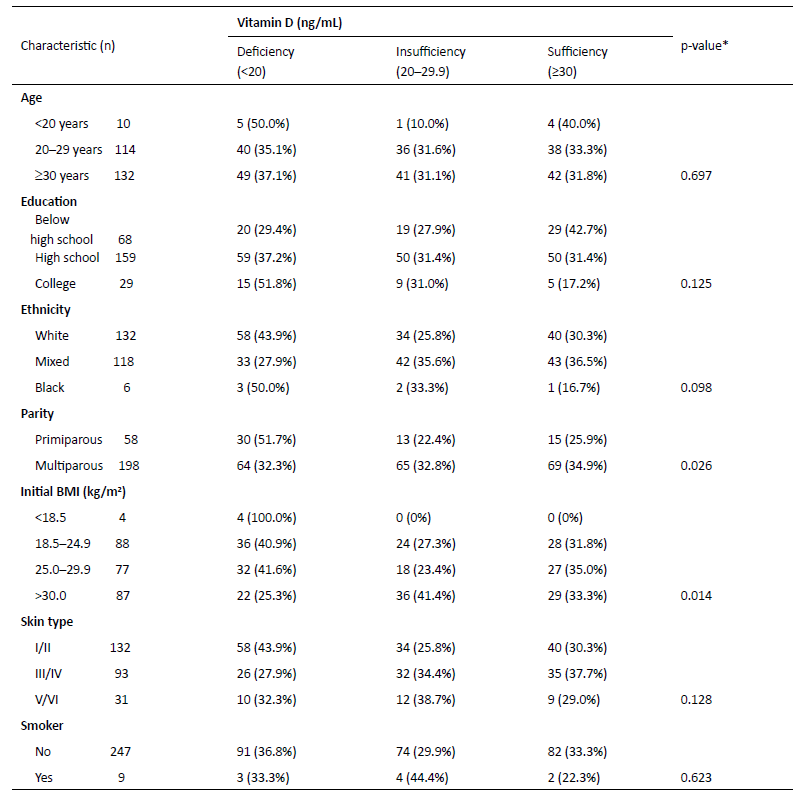
*p <0.05 based on Pearson’s X 2 test. BMI, body mass index
No association was found between education, ethnicity, skin type, BMI, and smoking and vitamin D sufficiency in Winter (Table 4). On the other hand, subjects aged ≥30 years had a significantly higher incidence of vitamin D sufficiency (p=0.032). Primiparous women had a higher incidence of vitamin D deficiency than multiparous women, but the difference was not statistically significant (p=0.079).
Table 4 Association between maternal characteristics and serum vitamin D levels during Winter in Brazil
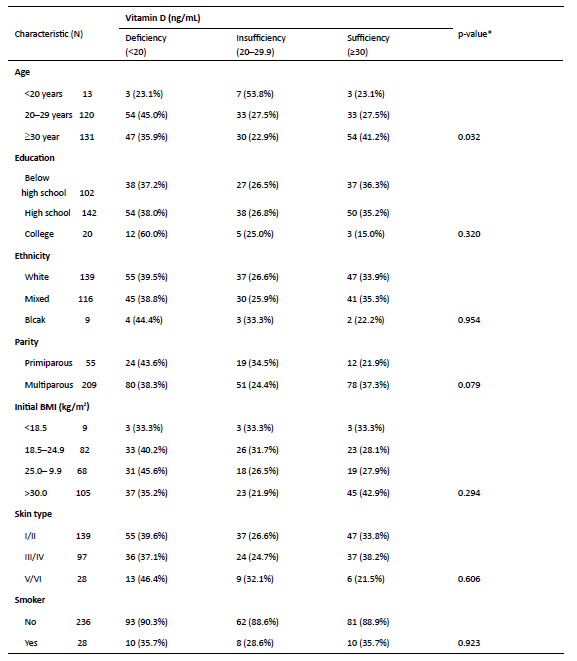
*p <0.05 based on Pearson’s X 2 test. BMI, body mass index
HIV+ women had significantly higher vitamin D levels than women in other disease groups (p=0.001), while women with GDM had significantly lower vitamin D levels than women with low-risk pregnancy or PE (Table 5).
Discussion
As far as the authors are aware, this is the first study of serum vitamin D levels in pregnant women in south Brazil and shows a high prevalence of hypovitaminosis D in the Summer months, which becomes worse in Winter.
Hypovitaminosis D during pregnancy has attracted much interest in recent years due to its association with gestational and neonatal complications. Around 1 billion people globally are estimated to have the condition.14
In a review including several countries, a high prevalence of hypovitaminosis D was reported globally in infants, children, adolescents, adults, and the elderly throughout the year, including in sunny countries.15 The highest prevalence was found in the Middle East. In most South American and African countries, there is not enough data.
Another review included seventeen studies on pregnant and lactating women, two from America, six from Europe, one from Africa, seven from Asia, and one from Oceania.15 The authors reported vitamin D insufficiency in 33% of pregnant women in the USA, 24% in Canada, 45% in Belgium, 35% in the United Kingdom, 44% in the Netherlands, 20% in Spain, and 77% in Germany. In addition, vitamin D deficiency was reported in 12% of women in Belgium, 4% in England, and 23% in the Netherlands. A single study in Africa reported that 1% of pregnant women presented with hypovitaminosis D. In Asia, the prevalence of hypovitaminosis D in pregnant women was found to be 90% in Turkey, 67% in Iran, 72% in Pakistan, 70─83% in Kuwait, 96% in India, and 69% in China. In Australia, vitamin D insufficiency was found in 48% and vitamin D deficiency in 15% of pregnant women.15 In the present study in tropical Brazil, the prevalence of vitamin D insufficiency and deficiency was high, with only 8.7% of subjects having sufficient vitamin D levels in Winter. Regardless of the season, more than half of subjects did not have adequate levels of this vitamin.
According to some authors, seasonality increases the risk of hypovitaminosis D in pregnancy, with a higher prevalence of vitamin D deficiency during Winter compared to Summer months.16,17 The results of this study support such findings. Serum vitamin D levels in pregnant women were significantly lower in Winter compared to Summer. The study evaluated pregnant women from the city of Curitiba and surrounding metropolitan region, located in the State of Paraná and in the southern region of Brazil, where Winter is most rigorous. Brazil is a country with continental dimensions, with latitudes between 3ºN and 33ºS. Curitiba is located at a latitude of 25ºS and at an altitude of 934 meters. Previous studies have shown that the latitude directly influences vitamin D levels, with the higher the latitude, the higher the prevalence of hypovitaminosis D.18
Some global health organizations recommend vitamin D supplementation during pregnancy.19,20 The World Health Organization 2020 update of the recommendations for vitamin D supplementation suggests that supplementation during pregnancy improves maternal vitamin D status and may reduce the risk of PE, low birthweight, and preterm birth. However, the evidence currently available to directly assess the benefits and harms of the use of vitamin D supplementation alone in pregnancy for improving maternal and infant health outcomes is limited.21 The American Academy of Pediatrics suggests monitoring serum 25(OH)D levels during gestation, but there is no consensus regarding the appropriate levels. The US Institute of Medicine considers 20 ng/mL to be adequate, although most researchers consider that ideal levels to be ≥30 ng/mL. The Endocrine Society recommends that pregnant women consume 1,500 to 2,000 (up to 4,000) IU/day, as this has been shown to be safe and effective.13 Each 100 IU of vitamin D administered raises serum 25(OH)D levels by about 1.0 ng/mL (range 0.7-1.1 ng/mL).22 The dose of vitamin D required to prevent or treat vitamin D deficiency has not been established. Some researchers suggest 1,000 IU/day, others 5,000 IU/week, and others a single dose of 200,000 IU or higher.22-25 The Brazilian Endocrinology and Metabolism Society recommends daily oral vitamin D supplementation in the dose of 600 IU/d for pregnant women in general, 600 to 1,000 IU/d for pregnant women aged 14 to 18 years, and 1,500 to 2,000 IU/d for pregnant women at risk aged over 18 years.26
Nourishment guidance should be provided to pregnant women, advising on the adoption of a diet rich in natural sources of vitamin D. Detailed next is the vitamin D uptake provided by 100 g of each of the following foods: fresh wild salmon (≈600-1000 UI of vitamin D3); fresh farmed salmon (≈100-250 UI of vitamin D3); canned sardines (≈300 UI de vitamin D3); canned mackerel (≈250 UI of vitamin D3); canned tuna (≈230 UI of vitamin D3); fresh shiitake mushrooms (≈100 UI of vitamin D2); sun-dried shiitake mushrooms (≈1600 UI of vitamin D2). Also importantly, 5 mL of cod liver oil provide ≈400-1000 UI of vitamin D3, and one unit of egg yolk provides ≈20 UI of vitamin D3.27
In Brazil, the assessment of serum of 25(OH)D levels is not part of routine prenatal care. However, in regions with a high prevalence of hypovitaminosis D, this assessment should be performed during gestation. Ideally, a woman who intends to become pregnant should have sufficient antenatal serum 25(OH)D levels. During pregnancy, levels should be periodically monitored in accordance with the pregnancy stage. Guidelines for the prevention or treatment of hypovitaminosis D during pregnancy through sun exposure and adequate nutrition, together with supplementation, if necessary, are required to avoid maternal-fetal complications and problems during the neonatal period, early childhood, or even adulthood. As there is no consensus regarding vitamin D supplementation during pregnancy, recommendations should be individualized according to serum 25(OH)D levels. When laboratory tests cannot be performed, sun exposure and adequate nutrition should be reinforced.
This study’s cross-sectional design and the impossibility of evaluating samples from the same pregnant woman in both seasons were study limitations that should be acknowledged.
In conclusion, this study documents the importance of assessing women’s serum vitamin D status during pregnancy, in particular considering seasonal effects, providing nutritional advice and recommending regular physical activity and adequate sun exposure, with or without vitamin D supplementation. Future studies are required to assess the need for vitamin D supplements during pregnancy and, if necessary, determine their most appropriate dose.
Authorship
Kadija Rahal Chrisostomo - Conceptualization; Data curation; Formal Analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization ; Writing - original draft; Writing - review & editing
Renato Nisihara - Project administration; Resources; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing
Caroline Vieira de Souza - Data curation; Formal Analysis; Investigation; Methodology; Resources; Software; Visualization
Eduardo Rahal Chrisostomo - Data curation; Formal Analysis; Investigation; Methodology; Resources; Software; Visualization
Jessica Fujie - Data curation; Formal Analysis; Investigation; Methodology; Resources; Software; Visualization
Jaime Kulak Junior - Project administration; Resources; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing