Introduction
Cystic fibrosis (CF) is a multisystemic autosomal recessive disease caused by mutations on the cystic fibrosis transmembrane conductance regulator (CFTR) gene, located on chromosome 7 and encoding the CFTR protein.1 Deficit or dysfunction of this protein limits mucociliary clearance and promotes chronic airway obstruction and anatomical changes in the bronchial tree, increasing susceptibility to respiratory infections.1-3) Respiratory viruses are often responsible for pulmonary exacerbations and increased morbimortality in children with CF.
Coronavirus disease 2019 (COVID-19) presentation may range from mild to severe, but is generally milder in children than in adults.4,5) However, the presence of comorbidities, as diabetes mellitus, hypertension, and chronic lung diseases (including CF), has been identified as a major risk factor for severe COVID-19.3-5 Early in the pandemic phase, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was expected to have a significant impact on patients’ respiratory status, prompting the recommendation for strict isolation measures for patients with CF. Due to these measures, only few cases of SARS-CoV-2 infection in patients with CF were reported worldwide.1,4 Detailed data of the clinical course of COVID-19 in children with CF is scarce.
The relief of restrictions and emergence of the Omicron variant led to the highest rates of COVID-19 infection seen in children, including those with CF.6
The aim of this study was to determine the incidence, clinical characteristics, and course of COVID-19 infection in children and adolescents with CF followed at a Portuguese reference centre.
Material and methods
A retrospective review of the medical records of children aged less than 18 years old followed at a Portuguese CF reference centre between March 2020 and March 2022 was conducted. The only inclusion criterion was a confirmed diagnosis of CF. Children were divided into two groups: Group 1, of children with documented SARS-CoV-2 infection, and Group 2, of children who never tested positive for SARS-CoV-2 during the observational period.
Data were extracted from patients’ clinical records after informed consent, and assent was obtained according to the national legislation when appropriate. Retrieved data included demographics, vaccination status against COVID-19, and clinical manifestations, treatment, and outcomes of the infection. A COVID-19-positive case was considered when the patient had positive antigen or reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 in a respiratory sample. COVID-19 severity was classified according to national guidelines.7) Children were considered fully vaccinated two weeks after receiving the second dose of the messenger RNA (mRNA) Pfizer-BioNTech COVID-19 vaccine.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 22. For group comparisons, the non-parametric two-tailed Mann-Whitney U test was used for continuous variables, and Fisher’s exact test was used for categorical variables, as appropriate. P-values <0.05 were considered statistically significant.
Results
Demographics of the study population
Among 30 children with CF followed at the study centre, 12 (40%) had a confirmed diagnosis of SARS-CoV-2 infection during the study period, in a total of 13 episodes (one adolescent was infected in two different occasions). All children were tested due to symptoms or during contact tracing. No case was detected during hospital admission for other reasons. The characteristics of the study population are depicted in Table 1.
Age, sex, CF mutations, percentage of pancreatic failure, and body mass index (BMI) did not differ between infected and non-infected children. The median best forced expiratory volume in one second (FEV1) within 12 months prior to infection for children over five years old was 87.1% (Q1-Q3: 83.6-88.5). One infected child met Leeds criteria for chronic infection with Pseudomonas aeruginosa.8 Although no patient had CF-related diabetes (CFRD), two (17%) presented an abnormal oral glucose tolerance test (OGTT). The two children under immunosuppressive therapy followed at the centre ─ one due to a previous lung transplant and the other due to steroid-resistant nephrotic syndrome ─ were not infected.
No statistically significant differences were found between groups for any of the studied variables.
Table 1 Characteristics of the CF study population, by SARS-CoV-2 infection status
| GROUP 1: Patients with confirmed SARS-CoV-2 infection (N=12) | GROUP 2: Patients without confirmed SARS-CoV-2 infection (N=18) | p-value | |
| Age, years: median (Q1-Q3) | 10.5 (6.5-16.25) | 12 (6-13) | 0.925* |
| Male (N, %) | 4 (33%) | 8 (44%) | 0.709** |
| F508del homozygous (N, %) | 6 (50%) | 5 (28%) | 0.266** |
| Pancreatic failure (N, %) | 10 (83%) | 10 (56%) | 0.235** |
| BMI percentile (median; Q1-Q3) | 50 (15-50) | 50 (22-60) | 0.400* |
| FEV1 (median; Q1-Q3) | 87.1% (83.6-88.5) | 97%; (82.7-102.4) | 0.190* |
| Chronic P. aeruginosa infection | 1 (8%) | 2 (11%) | 1.000** |
| CFRD (N, %) | 0 | 1 (5.6%) | 1.000** |
| Abnormal OGTT (N, %) | 2 (17%) | 4 (22%) | 1.000** |
| Immunosuppressive therapy (N, %) | 0 | 2 (11%) | 0.503** |
BMI - body mass index; CFRD - cystic fibrosis-related diabetes, FEV1- forced expiratory volume in one second; N - number; OGTT - oral glucose tolerance test, Q- quintile; SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2. * Mann-Whitney U test. ** Fisher’s exact test
Clinical manifestations, treatment, and outcomes of COVID-19 infection in the CF study population
Most cases (69%) occurred between January and March 2022, when the Omicron variant was predominant in Portugal. Three cases (23%) occurred during the first months of 2021 and only one case was reported in 2020.
All cases but one referred to mild disease. Three children (23%) were asymptomatic and 9 (69%) presented with mild symptoms, as fever, rhinorrhea, increased cough, or myalgia, which persisted for one to two days and were easily managed with paracetamol or ibuprofen. Only one patient presented with moderate disease, specifically pneumonia without hypoxemia7. This was a 12-year-old boy with homozygous F508del mutation, pancreatic failure, and previous FEV1 of 93.7%. Of notice, his BMI percentile was 3. At the time of infection, the boy had received one dose of the mRNA Pfizer-BioNTech COVID-19 vaccine. He was admitted to the Emergency Room of the local hospital with fever, rhinorrhea, cough, chest pain, and vomiting (Day 1). Blood analysis revealed lymphopenia (300/μL), C-reactive protein of 25.2 mg/L, and sedimentation rate of 32 mm/h. Chest X-ray showed an infiltrate in the lingual segment of the left upper lobe. PCR test for SARS-CoV-2 was positive, blood culture was sterile, and sputum culture was not performed due to inadequate sample collection. The boy was hospitalized for clinical surveillance and intravenous amoxicillin-clavulanate therapy. He was discharge on Day 3, maintaining oral amoxicillin-clavulanate for 7 days.
No patient required supplementary oxygen therapy or admission to the Intensive Care Unit, and no mortality was reported.
After two months of follow-up, one patient complained of mild cough and another of fatigue that were not explained by other exacerbations, and none reported breathlessness or neurologic symptoms. Symptoms subsided after three months in both patients. All remaining 10 patients (83%), including the one hospitalized, fully recovered after COVID acute phase.
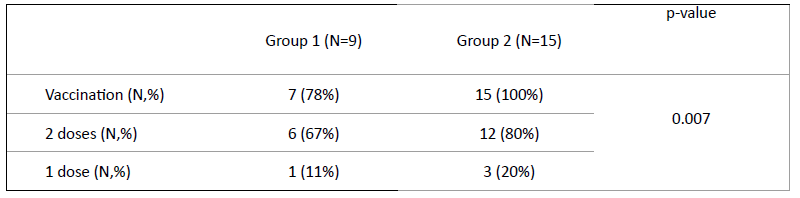
COVID-19 vaccination status of the study population
Nine of the 12 patients with SARS-CoV-2 infection met national criteria for COVID-19 vaccination, according to which the mRNA Pfizer-BioNTech 2-dose schedule is recommended for all children over the age of 5 years. At the time of infection, seven children (78%) had been previously vaccinated, six (67%) had been fully vaccinated with both doses, and one (11%) had received only one vaccine dose. Fifteen of the 18 patients in Group 2 met COVID-19 vaccination criteria, and all were vaccinated (80% with two and 20% with one vaccine dose; Table 2).
Discussion
Although previous reports classified chronic lung disease, like CF, as a major risk factor for severe COVID-19, in the present series, most CF children infected with SARS-CoV-2 (92%) were asymptomatic or presented mild disease that could be managed at home and did not require supplemental oxygen therapy or hospitalization.3 Only one patient was treated with intravenous antibiotic, a standard practice for moderate-to-severe CF respiratory exacerbations, but none required antiviral medication or experimental treatments for COVID-19. These data were similar to those reported in recent international studies showing that the course of SARS-CoV-2 infection in CF patients is not as severe as initially predicted.3-5 The predominance of COVID-19 cases during the winter months of 2022, when the Omicron variant prevailed, may justify the prevalence of mild disease in this series, since this variant is associated with less severe symptoms.6
The demographics and clinical characteristics of the present study cohort are similar to those reported in international series.3),(4 Recent studies show that the main risk factors for severe COVID-19 in patients with CF are advanced age, lung transplantation, low FEV1, and CF-related diabetes.3)-(5 In this study, SARS-CoV-2-infected patients had preserved lung function, and none of the children with CF presenting with risk factors (immunosuppression, lung transplantation, or CFRD) had documented SARS-CoV-2 infection, which may have contributed to the observed benign course observed in this series. CF patients are used to being compliant with protective measures, including using face-mask and maintaining social distancing and adequate hand hygiene, which may have contributed to the low incidence of SARS-CoV-2 infections found. Patients with additional risk factors were not infected, which may be explained by better infection control measures in this groups of patients.
Interestingly, the only child in this study who was hospitalized ─ due to non-complicated pneumonia with no need for supplemental oxygen ─ had the lowest BMI of this series. In a recent international observational study, BMI Z-scores were lower in hospitalized CF children then in those managed in community setting.4
One reason that may explain why not all patients had received the two vaccine doses is the fact that the vaccination campaign for children younger than 12 years old only started during the emergence of the Omicron variant, as most parents were willing to vaccinate their children. This fact, together with the estimated lower protection rate of the vaccine against the Omicron variant, could have contributed to the high SARS-CoV-2 infection numbers observed.6 However, amongst children who fulfilled national criteria for vaccination, there was a higher rate of documented infection in unvaccinated or incompletely vaccinated compared to fully vaccinated children with CF. Furthermore, patients with moderate disease were not vaccinated with the two recommended doses.
This study has important limitations that should be addressed. The first is its small sample size, explained by the fact that only one pediatric reference centre was included. The second is its retrospective data collection, which may have resulted in underreporting of some symptoms, although this did not impact the analysis of disease severity. Due to the difficulty in performing lung function tests during the pandemic, especially in infected patients, it was not possible to assess whether there were changes in FEV1 during the acute phase of the infection. It is possible that the number of asymptomatic patients is underestimated, as national testing criteria during the study period included the presence of symptoms or close contact with a confirmed case, and there were no recommendations for routine SARS-CoV-2 testing. This was also a limitation in the few similar studies published in the literature. Finally, it should be noted that COVID-19 is a novel disease, and thereby scientific knowledge is constantly being updated, especially regarding treatment and vaccination.
Conclusions
In agreement with other studies, the present analysis showed that patients with CF usually present mild COVID-19 disease, with no need for hospitalization or oxygen therapy and no long post-COVID symptoms of concern. The follow-up of these patients is crucial to assess the impact of COVID-19 on lung function and the emergence of long-term complications.