Introduction
Pediatric stroke occurs by focal interruption of cerebral blood flow, confirmed by neuroimaging.1-3 It encompasses ischemic and hemorrhagic stroke and produces neurological deficits that persist for more than 24 hours.1-3 Although rare, it is more common than thought and often underdiagnosed.4,5Factors contributing to the underdiagnosis include a low level of suspicion by parents and health professionals, subtle and non-specific symptoms mimicking other more common diseases in pediatric age, and difficulties performing neuroimaging assessment due to the need for sedation.6
In pediatric age, stroke can be classified as perinatal − when occurring from 28 weeks of gestation to 28 days of life − or pediatric − when occurring from 28 days to 18 years of age.5
The causes and risk factors for stroke in pediatric age differ from adults.7-10 While adult ischemic and hemorrhagic stroke is commonly associated with atherosclerotic disease and cardiovascular risk factors, pediatric ischemic stroke is mainly related to arteriopathies, followed by hematologic and heart diseases7-10. Brain tumors, arteriovenous malformations, cavernomas, or aneurysms are the main contributors to cerebral hemorrhagic events in the pediatric population7-10. Another big difference between stroke in adults and children is that, contrary to what happens in adults, in pediatric age deficits may not be evident until some point in the course of psychomotor development7-10. Prompt recognition and early treatment are critical for optimizing functional outcomes and decreasing event recurrence rate6. The literature reports a mean time of 28.5 hours between first symptoms and demand for medical care and 35.7 hours between medical care and diagnosis.6 Therefore, it is key to highlight the relevance of stroke in the development of skills and fulfillment of individuals’ full potential.
AIM
To characterize the pediatric population with stroke followed in the Physical and Rehabilitation Medicine (PRM) consultation in a tertiary center and conduct a literature review on pediatric stroke rehabilitation care.
Material and methods
A retrospective study was carried out between November 2017 and October 2018 (12 months) in stroke patients followed at a pediatric PRM consultation, at the first PRM observation. Stroke was defined as cerebral blood flow interruption by rupture or blood vessel occlusion confirmed by neuroimaging. Patients’ gender, age, type of stroke (ischemic vs hemorrhagic and perinatal vs pediatric), vascular territory, risk factors, functionality (assessed through the Gross Motor Function Classification System [GMFCS] and the Manual Ability Classification System [MACS]), and stroke recurrence and mortality in the specified time period were assessed. Data were processed using the Statistical Package for the Social Sciences (SPSS). Reassessment consultation was carried out according to patients’ needs and was not included in the present study.
A bibliographic search was conducted on PubMed and Medline databases using the keywords ‘stroke’, ‘pediatric’, ‘perinatal’, ‘neuroplasticity’, ‘functionality’, and ‘rehabilitation’. Inclusion criteria comprised meta-analysis, systematic review, and review type of studies, written in Portuguese and English languages, and focusing human studies.
Discussion
Study population
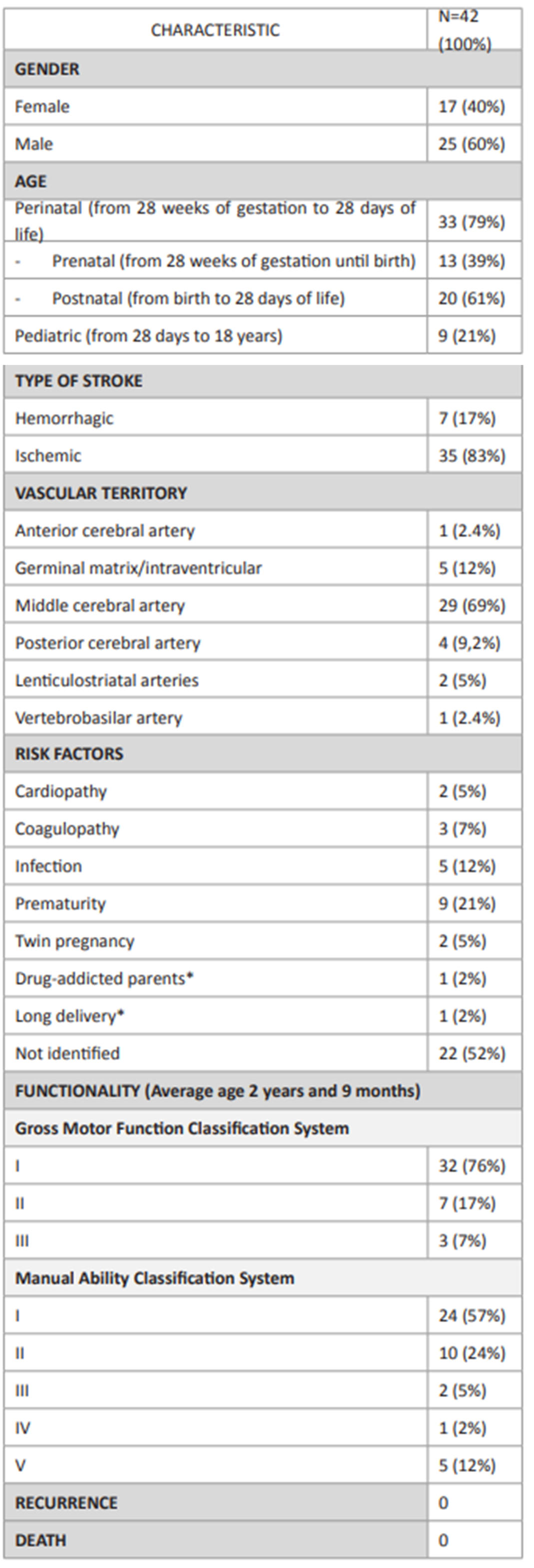
This study included 42 patients, 25 of whom were males and 17 females. Thirty-three (79%) reported stroke at perinatal and 13 at prenatal age (Tables 1and2).
Thirty-five cases of ischemic stroke were reported, compared to only seven cases (17%) of hemorrhagic stroke. The territory supplied by the middle cerebral artery (MCA) was mostly affected, representing 29 cases (69%), followed by the germinal matrix (with intra-ventricular hemorrhage) in five cases (12%), the posterior cerebral artery in four (9%), the lenticulostriatal arteries in two (5%), and the anterior cerebral artery and vertebrobasilar arteries in one each (2.4%). The most common symptom was hemiparesis.
Regarding risk factors, 50% of patients had known risk factors for stroke: prematurity in 21%, infectious diseases (varicella-zoster virus, human immunodeficiency virus, umbilical vasculitis) in 12%, coagulopathy (factor V Leiden mutation) in 7%, twin pregnancy in 5%, and cardiopathy (atrial septal defects) in 5%. Drug-addicted parents (2%) and long delivery (2%) were additionally identified as risk factors.
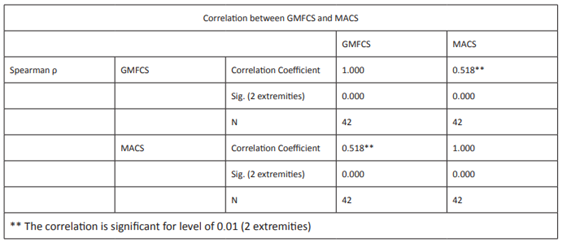
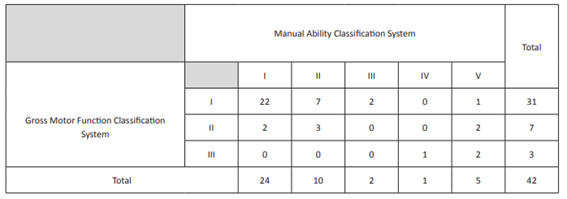
The study cohort had an average age of two years and nine months (ranging between one month and eleven years) and 76% had GMFCS I, 17% GMFCS II, and 7% GMFCS III in functional assessment. Regarding upper limb, 57% had MACS I, 24% MACS II, 12% MACS V, 5% MACS III, and 2% MACS IV. Spearman’s correlation coefficient was 0.518, showing a positive correlation between both scales (Table 3). No stroke recurrences or deaths were reported during the study period.
Table 1 Characteristics of the study population
Legend: * Additional risk factor to the one previously described.
Table 2 Gross Motor Function Classification System and Manual Ability Classification System cross table
Literature review
Stroke is one of the most relevant morbidity causes and one of the top ten death causes in children.5 Sixty percent of those who survive have permanent neurological deficits, most often motor (hemiparesis/hemiplegia), but also cognitive, sensory, epileptiform activity, speech and communication disorders, visual disturbances, low attention, and behavioral problems, all associated with decreased QoL. As many as 15% of children die and 35% remain neurologically normal.11 Formal psychometric or neuropsychological assessment is required in the follow-up of stroke patients, to assess cognitive impairment and help plan a therapeutic and educational plan adjusted to the children’s needs.12 These exams were not available in the present study.
Prompt recognition and early treatment within the first hours/days after the stroke is critical in optimizing functional outcomes and decreasing event recurrence. Preference for using one hand over the other may represent an important warning sign in children younger than one year and should raise suspicion of a stroke.
Infancy (especially early infancy) is the period with the greatest cerebral plasticity, which allows a better prognosis following a stroke than in adults if the condition is correctly guided in both acute and subacute phases. Neuroplasticity is one of the most prominent central nervous system features, enabling skill learning and memorization by reorganization of neural networks in response to environmental stimuli. It is usually adaptive and beneficial, but can also be maladaptive. The mechanisms involved in this process include neurogenesis, programmed cell death, and synaptic activity-dependent plasticity. In the long term, repeated synapses stimulation causes neurotransmitter potentiation or depression. Adaptive neuroplasticity includes the reorganization of cortical maps after constraint-induced movement therapy to improve hemiparesis caused by stroke. This form of plasticity is associated with brain structural and functional changes, which can be detected by magnetic resonance imaging, positron emission tomography, and transcranial magnetic stimulation (TMS). TMS and other forms of brain stimulation are also experimentally used to improve brain plasticity and restore function.13
Physiatric treatment modulates neuroplasticity based on motor learning principles: repetition, functional relevance (specific task), tolerance, and gains.14 The Canadian stroke best practice recommendations on stroke rehabilitation include a pediatric stroke section. Given the large geographical area covered by our central hospital, many children undergo the rehabilitation program in their residential areas. Therefore, it is not possible to know exactly what type, duration, and intensity of treatments are carried out. However, according to the child’s assessment and parents’ experience, the most relevant areas of action are outlined at the PRM hospital consultation and include physical, occupational, and speech therapy. With the child’s growth, development, and additional physical and cognitive demands, deficits may emerge and the rehabilitation plan should be adjusted.15 Follow-up is maintained with the appropriate frequency for each case, to evaluate gains, therapeutic adjustment, and prescription of support products, if necessary. This may include a splint for positioning the upper limb, ankle-foot orthosis, or wheelchair. According to best practice recommendations, all patients with stroke should receive rehabilitation therapy as early as possible once they are stable. If there are no contraindications, mobilization should start between 24h and 48h of stroke onset. Patients should receive direct task-specific therapy three hours per day, five days a week (Evidence Level C). More therapy results in better outcomes (Evidence Level A).15
For outpatients, therapy should be provided for a minimum of 45 minutes per day (Evidence Level B), two to five days per week, based on individual patient needs and goals (Evidence Level A), for at least eight weeks (Evidence Level C). Patients and families should be involved in management and goal setting from the beginning and throughout the process (Evidence Level A).15
Motion restriction therapy is the primary approach in the treatment of hemiparesis (Evidence Level A). It has the strongest evidence in upper limb intervention, greater spontaneous limb use, and higher motor function score and maintenance gains from three weeks to six months of follow-up. This intervention is carried out in sessions and at home upon parent/caregiver’s education. The type of restriction used must be safe, comfortable, and adapted to each child - sling, mitt/sock, orthosis, and immobilization by third parties.14
Bimanual therapy allows task practice/completion with functionally significant objectives and bimanual coordination improvement. The combination of motion restriction therapy with bimanual therapy results in better integration of the affected side into the body scheme.
If there is focal upper and/or lower limb spasticity or dystonia, botulinum toxin type A may be used for chemodenervation to increase the range of motion and decrease pain (Evidence levels: Early - Level C; Late - Level A).12,15
Mirror therapy is another tool that provides cortical response to performance-like action/movement observation. There is increasing evidence that mirror neuronal system is an important component of motor learning. It is activated during action and observation. It may help to improve upper extremity motor function and daily living activities (DLA) (Evidence Level A).15
Robotic and computer engineering have also evolved to provide new tools and treatments to help patients and improve their rehabilitation. Robotic therapy is based on functional games, especially directed at gait training, but evidence of its benefit remains low.
Virtual reality provides an environment, experience, and activity that is more stimulating and attractive to children. It promotes cortical integration and reorganization, resulting in functional improvement.
Non-invasive cerebral stimulation modulates fundamental nervous system properties, namely conduction speed and response to stimuli. It can be repetitive or continuous and activate or inhibit neuronal activity in target regions of the cerebral cortex but is not yet approved for pediatric use.
These therapies should be integrated into the child’s rehabilitation program, as they complement each other, to maximize functional capacity in pediatric age.
DLA training should be introduced early to encourage autonomy. If the dominant upper limb is non-functional, lateral transfer should be initiated. Compensation strategies or arrangements should be made available. Raising family awareness not to replace the child in tasks and activities is of utmost importance; the child should be encouraged to perform them despite of his/her limitation.
Language re-education should be part of treatment in cases of developmental aphasia − also known as dysphasia (pre-linguistic injury) −, given the early age at which events often occur. Exercises aimed at attention, perception, orientation, naming, evocation, sentence construction, and reading and writing should be prescribed, when applicable.
Speech re-education, verbal articulation disorders, dysarthria, drool control, and/or changes in suction and mastication involve orofacial motor training and respiratory control training.
Swallowing re-education is another important treatment aspect in cases of dysphagia. Correct cephalic and trunk positioning, adequate food consistency and volume, oropharyngeal cleansing strategies, nasogastric catheter, and percutaneous gastrostomy are measures that can be implemented in cases of dysphagia.
But the most important measure is to promote the transfer of skills acquired in therapy to patients’ daily routine (Evidence Level A).15
Discussion
This study’s results agree with data in the literature regarding higher stroke incidence in males and perinatal age (estimated incidence of 1/3,500 births).1-3,5,16-19After the first month of life, stroke is reported in 13/100,000 children per year.5 At perinatal age, events are ischemic in 80% of cases.3,5 After the first month of life, 50% of events are ischemic and 50% are hemorrhagic. 3,5 In the present cohort, stroke in perinatal age prevailed, with ischemic stroke having the highest expression (80% of cases). The most affected vascular territory was the MCA. Previous known risk factors were identified in 50% of cases, usually prematurity, infectious conditions, cardiopathy, or coagulopathy. According to the literature, up to 75% of patients present with more than one risk factor.6
Regarding functionality, 75% of this patient cohort had grade I GMFCS and about 50% grade I MACS, with results pointing towards a correlation between GMFCS and MACS. Therefore, it seems that better lower limb functionality (namely in gait capacity) is related to better upper limb functionality, with better limb integration in daily living activities, performance, and manual ability. However, this relationship is not always verified, as patients with GMFCS I may present MACS V. No studies were found in the literature analyzing functionality, but two studies were found addressing quality of life (QoL) in pediatric age.20,21 In the first, QoL was evaluated in 78 children/adolescents, 39 of whom with stroke and 39 with no cerebrovascular event (control group). The Pediatric Quality of Life Inventory PedsQL was used, according to the perception of the responsible person and children/adolescents themselves. The study reported lower QoL scores in the post-stroke group20. In the second study, 49 children were recruited and their QoL was rated by themselves, parents, and teachers. Results showed that health-related QoL in the physical, emotional, social, school, and cognitive domains was significantly lower in children that suffered a stroke compared to healthy ones.21
In the present cohort, cerebrovascular events predominated at perinatal age and no stroke recurred. The literature reports a recurrence of perinatal stroke lower than 1% and of pediatric stroke of 15-18%.5 Also no patient died during the study period, but the literature reports mortality of 7−28%.4
As this was a 12-month retrospective study, patients did not have the same evolution and their observation depended on the timing of referral to Physiatry from other medical specialties. Besides stroke extension, the child’s environment also strongly contributes to the outcome. Therefore, all sequelae should be treated to minimize detrimental consequences and allow the best psychomotor development.
Conclusions
Although uncommon in pediatric age, stroke is a major cause of morbidity and mortality. Diagnosis is difficult, as symptoms are often subtle and mimic other more frequent diseases in this age group. Timely referral to the pediatric physiatrist allows child integration into a rehabilitation program, to optimize neuroplasticity and participation in several activities. The effectiveness of early intervention is particularly relevant in children and has been shown to increase educational and developmental gains.
Evidence-based guidance regarding rehabilitation is still lacking in this population. The definition of key areas for future research and multicenter collaborations is necessary to improve the outcomes of pediatric stroke survivors.