Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.29 no.1 Porto jan. 2020
https://doi.org/10.25753/BirthGrowthMJ.v29.i1.15101
CASE REPORTS | CASOS CLÍNICOS
Treatment interruption in small for age term-born children
Interrupção do tratamento em criança nascida a termo pequena para idade gestacional
Louise CominatoI, Alexandre FrascinoI
I. Endocrinology Unit, Instituto da Criança, Hospital das Clínicas Faculdade de Medicina da Universidade de São Paulo. 05403-000 São Paulo, Brasil. aledefra@gmail.com; louise.cominato@hotmail.com
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Small-for-gestational-age children have been associated with up to 20% delayed growth and delayed development, increased childhood morbimortality, and poor quality of life. Prepubertal growth hormone administration results in increased growth and musculoskeletal development. The present case report illustrates the negative impact of growth hormone disruption in a 6-year-old patient, resulting in impaired growth and development. Close clinical and laboratory monitoring is recommended for small-for-gestational-age children receiving growth hormone treatment, and family counselling should be encouraged.
Keywords: growth hormone; growth impairment; small-for-gestational-age
RESUMO
Crianças nascidas pequenas para a idade gestacional têm sido associadas a atrasos no crescimento em até 20% e atrasos no desenvolvimento, aumento da morbimortalidade na infância e pior qualidade de vida. A administração pré-pubertária de hormona de crescimento resulta em aumento do crescimento e do desenvolvimento musculoesquelético. O presente caso ilustra os impactos negativos da interrupção da administração de hormona de crescimento numa criança de 8 anos de idade, com consequente comprometimento do crescimento e desenvolvimento. É aconselhável manter uma monitorização clínica e laboratorial periódica das crianças pequenas para a idade gestacional em tratamento com hormona de crescimento e incentivar aconselhamento familiar para evitar a interrupção terapêutica.
Palavras-chave: comprometimento do crescimento; hormona do crescimento; pequeno para a idade gestacional
Introduction
Term children born small-for-gestational-age (SGA) have twice decreased standard deviation (Z-score) birthweight and length for gestational age.1 These children have increased risk of childhood morbimortality and poor quality of life.2 Although several cases remain unreported, according to the United Nations Children’s Fund (UNICEF) State of the World’s Children report, SGA affects 14% of term deliveries in developing countries.3
Fetal human growth results from the combination of genetics, fetal and mother health, and nutritional availability, with the unbalance of any of these factors potentially impairing fetal growth. Intrauterine growth restriction is the commonest cause of impaired fetal growth.4
SGA individuals are prone to develop cardiorespiratory diseases, musculoskeletal growth impairment, obesity, immunologic deficits, and cognitive impairment.4 An increasing number of SGA children currently reach adult age, highlighting the need for adequate clinical follow-up for this subgroup of patients.5 This case report describes the long-term follow-up of a SGA patient receiving growth hormone (GH), with improved outcomes.
Case presentation
An eight-year, six-month-old boy with pale skin, born and raised in São Paulo, presented accompanied by his parents complaining of short stature and retarded growth. Parents reported that the child grew less than expected since the first year of life and was smaller than siblings and schoolmates. No medical follow-up was undertaken in the last four years.
Medical history included term birth through vaginal delivery, 2.250 g birthweight (Z-score: -2.19), and 45 cm length (Z-score: -1.89), with no disabilities and normal psychomotor development. Parents denied gestational complications, drug or alcohol abuse, and smoking. No history of continuous medical drugs, surgeries, or previous relevant diseases was reported and both parents had normal stature.
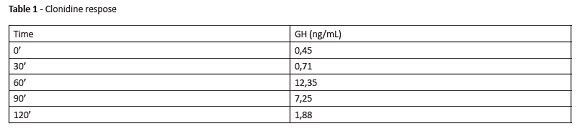
Physical evaluation revealed good general condition. The child was hydrated, normotensive, afebrile, and had no cognitive alterations. Thoracoabdominal examination was normal. The boy was prepubertal, with no syndromic stigmas, weighted 21.2 kg (Z-score: -1.7) and was 118-cm height (Z-score: -2.0). Laboratory assessments revealed normal venous blood gases, fasting blood glucose, thyroid-stimulating hormone (TSH), somatomedin (IGF-1), free thyroxine (free T4), total calcium, phosphorus, alkaline phosphatase, and anti-endomysium. Magnetic resonance imaging (MRI) of the pituitary gland showed preserved anatomical structures. Results of GH stimulation test with clonidine are described in Table 1.
After data collection, diagnostic hypothesis included slow growth rate due to SGA condition. Differential diagnosis included hormonal deficiencies and chronic diseases. GH (0.15 U/kg/day) subcutaneous injection was administered, and growth rate monitored every month.
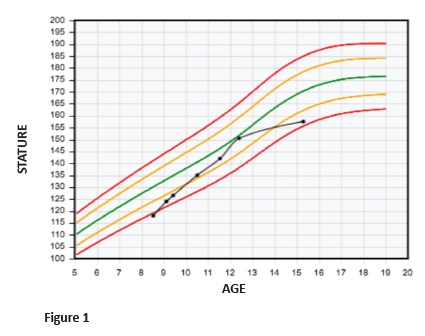
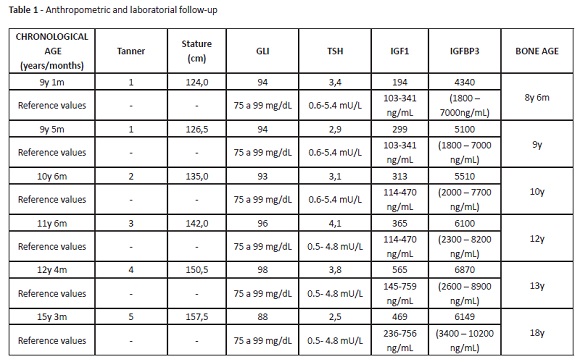
The patient evolved uneventfully, with good response to hormonal treatment. Discomfort or pain during treatment were not reported. Hormonal treatment provided a good growth rate, ranging from seven to nine cm/year (Figure 1). Stature monitoring and laboratory assessments are described in Table 2. Hormonal treatment was disrupted at the age of 14 due to patient absence.
Follow-up
Although weight and body mass index were adequate for age, three years after treatment disruption the patient complained of slow growth rate. At this time, it was not possible to continue hormonal treatment due to advanced bone age.
Discussion
Small-for-gestational-age (SGA) children may be term or pre-term. Diagnosis can result from pregnancy complications, maternal health impairment, obesity, drug or alcohol abuse, smoking, and/or chronic diseases.6-10 Fetal growth may also be influenced by an inherited predisposition.4 SGA is associated with increased risk of chronic diseases, being strongly correlated with metabolic syndrome and cardiovascular disease in adult life.11
Most live SGA newborns can recover from their weight and height deficits within the first years of life. Nevertheless, up to 20% of individuals will present deficient growth and short stature after puberty.12
GH is an indicated treatment for growth recovery in SGA children without catch-up growth in the first years of life. It has been acknowledged and approved in 2001 by the US Food and Drug Administration (FDA) for SGA children whose stature remains two standard deviation (-2 SD) below the mean for age and sex, starting at two years of age. In Europe, SGA treatment with GH was approved by the Committee for Proprietary Medicinal Products in 2003 and is currently indicated for children with the age of four with a deficit of less than 2.5 SD. It is also indicated when the child has a low growth rate or height below 1 SD of the familiar stature target.13 In this clinical case, the child had 2.250 g (Z-score: -2.19) at birth, being classified as SGA. Other causes of short stature were excluded after laboratory examinations and MRI of the pituitary gland. The patient’s short stature could have been diagnosed earlier, allowing to start GH treatment between the age of two and four and potentially resulting in a more favorable outcome.
Height and age at puberty onset, as well as the magnitude and duration of pubertal growth, are important factors influencing final height. Pubertal studies in SGA are controversial, but most authors seem to agree that puberty is initiated within the normal range, although relatively early for their short stature.13 At eight years and six months, this patient was 118-cm height. At nine years and five months, when puberty started, he was 126.5-cm height. At ten years and six months, a testicular increase was already apparent (Tanner 2), the boy was 136.5-cm height and had a bone age of ten years old. At the age of 14, and despite guidelines and medical counselling for treatment maintenance until the end of puberty, GH treatment was disrupted. Although the patient achieved a satisfactory stature, he did not achieve his target height.
Precocious puberty is also a concern in SGA children.13 At the age of ten, pubertal axis and puberty blockage with gonadotropin-releasing hormone (GnRH) analogues could have been evaluated. Instead, due to poor attendance, the patient only returned after one year, at 11 years and six months (142.0 cm of height, Tanner 3, bone age of 12 years). In view of this evolution, it was decided to only maintain GH at the dose of 0.15 U/kg/d. Although aromatase inhibitors could have been administered to block the puberty axis, this strategy was not pursued, as it is not approved.
Regarding bone age, the patient had closed epiphyseal extremities at the age of 18, and GH administration was suspended. When other causes of short stature and secondary comorbidities or cancer are excluded, hormonal treatment at early age with close follow-up remains an important preventive strategy for reducing the incidence of chronic diseases and improving quality of life in adulthood.14
Conclusion
Approximately 10% of all children born SGA fail to achieve sufficient catch-up growth and remain with short stature throughout childhood and adult life. An adequate spurt puberty growth is essential to guarantee a satisfactory final height in these children. In the present case, patient’s final height was impaired due to treatment drop out during puberty. Close monitoring and follow-up during childhood and adolescence are important to optimize treatment adherence and maintenance.
REFERENCES
1. Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, et al. Premature birth and later insulin resistance. N Engl J Med. 2004; 351:2179-86. [ Links ]
2. Griffin IJ, Lee HC, Profit J, Tancedi DJ. The smallest of the small: short-term outcomes of profoundly growth restricted and profoundly low birth weight preterm infants. J Perinatol. 2015; 35:503-10. [ Links ]
3. Boguszewski MC, Mericq V, Bergada I, Damiani D, Belgorosky A, Gunczler P, et al. Latin American consensus: children born small for gestational age. BMC Pediatr. 2011; 11:66. [ Links ]
4. Stalman SE, Solanky N, Ishida M, Aleman-Charlet C, Abu-Amero S, Alders M, et al. Genetic Analyses in Small for Gestational Age Newborns. J Clin Endocrinol Metab. 2018; 103:917-25. [ Links ]
5. Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013; 1:e26-36. [ Links ]
6. Taha TE, Dadabhai SS, Rahman MH, Sun J, Kumwenda J, Kumwenda NI. Trends in birth weight and gestational age for infants born to HIV-infected, antiretroviral treatment-naive women in Malawi. Pediatr Infect Dis J. 2012; 31:481-6. [ Links ]
7. Spracklen CN, Ryckman KK, Harland K, Saftlas AF. Effects of smoking and preeclampsia on birth weight for gestational age. J Matern Fetal Neonatal Med. 2015; 28:679-84. [ Links ]
8. Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol. 2015; 125:566-75. [ Links ]
9. Gauthier TW, Guidot DM, Kelleman MS, McCracken CE, Brown LA. Maternal Alcohol Use During Pregnancy and Associated Morbidities in Very Low Birth Weight Newborns. Am J Med Sci. 2016; 352:368-75. [ Links ]
10. Puertas A, Magan-Fernandez A, Blanc V, Revelles L, O’Valle F, Pozo E, et al. Association of periodontitis with preterm birth and low birth weight: a comprehensive review. J Matern Fetal Neonatal Med. 2018; 31:597-602. [ Links ]
11. Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007; 92:804-10. [ Links ]
12. Albertsson-Wikland K, Karlberg J. Natural growth in children born SGA with and without catch up growth. Horm Res. 2003; 59:129. [ Links ]
13. Boonstra V, van Pareren Y, Mulder P, Hokken-Koelega A. Puberty in growth hormone-treated children born small for gestational age (SGA). J Clin Endocrinol Metab. 2003; 88:5753-8. [ Links ]
14. Methusalemsdottir HF, Egilson S, Guethmundsdottir R, Valdimarsdottir UA, Georgsdottir I. Quality of life of adolescents born with extremely low birth weight. Acta Paediatr. 2013; 102:597-601. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Louise Cominato
Endocrinology Unit
Instituto da Criança
Hospital das Clínicas Faculdade de Medicina
Universidade de São Paulo
Email: louise.cominato@hotmail.com
Received for publication: 26.09.2018
Accepted in revised form: 12.06.2019