Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.26 no.1 Porto mar. 2017
ARTIGOS ORIGINAIS | ORIGINAL ARTICLES
Brain Magnetic Resonance evaluation and pubertal development variations among female adolescents
Tradução neuroimagiológica dos ritmos de maturação pubertária em adolescentes do sexo feminino
Paula FonsecaI; Daniela SeixasII, III; Helena FonsecaIV; Sofia BrandãoV; Manuel FontouraVI
I Department of Pediatrics, Unidade de Vila Nova Famalicão, Centro Hospitalar Médio Ave. 4761-917 Vila Nova de Famalicão, Portugal. paulamrfonseca@hotmail.com
II Department of Experimental Biology, Faculdade de Medicina, Universidade do Porto. 4200-319 Porto, Portugal. seixas@med.up.pt
III Department of Imaging, Centro Hospitalar de Vila Nova de Gaia/Espinho. 4434-502 Vila Nova de Gaia, Portugal. dseixas@med.up.pt
IV Department of Pediatrics, Faculdade de Medicina, Universidade de Lisboa. 1649-028 Lisboa, Portugal. helenaregalofonseca@gmail.com
V Magnetic Ressonance Unit, Centro Hospitalar São João. 4200-319 Porto, Portugal. sofia.brand@gmail.com
VI Department of Pediatrics, Faculdade de Medicina, Universidade do Porto. 4200-319 Porto, Portugal. manuelfontoura@hotmail.com
ABSTRACT
Aim: There is evidence that adolescents with distinct rhythms of pubertal maturation have different psychosocial behaviours. However, a relation between pubertal maturation and the development of the prefrontal cortex and limbic system was not yet established. The aim of this study was to apply brain magnetic resonance imaging to investigate brain development of teenagers with similar age but distinct timing of pubertal maturation.
Methods: We compared the hormonal profiles and brain structure and volume using magnetic resonance imaging of two groups of 14 year-old girls, four with an early pubertal development (group 1) and three with an average pubertal development (group 2).
Results: The age at onset of puberty was the only biological variable that distinguished the two groups (p = 0,05). There were no differences regarding global brain volume. However, early-maturers showed a tendency for an increased volume of all subcortical structures except for nucleus accumbens.
Conclusion: To our knowledge, this is the first work to address the relation of pubertal maturation timing and central nervous system development using brain magnetic resonance imaging. The observed tendency for an increased volume of the subcortical structures may be related to a possible delayed development of the nucleus accumbens in early-maturers, and may explain the increased vulnerability of this group to risk behaviours.
Keywords: Adolescence; brain development; magnetic resonance imaging; neuroimaging; puberty
RESUMO
Introdução: Há evidência que adolescentes com a mesma idade cronológica mas com estádios pubertários distintos têm comportamentos psicossociais diferentes. Desconhece-se, no entanto, se existe alguma relação entre o ritmo de maturação pubertária e o desenvolvimento do córtex pré-frontal e sistema límbico.
Objetivo: Pretendeu-se com este trabalho avaliar através de ressonância magnética, o desenvolvimento cerebral de adolescentes do sexo feminino com a mesma idade cronológica mas com distintos ritmos de maturação pubertária.
Material e Métodos: Avaliaram-se parâmetros hormonais e imagiológicos (idade óssea, ultrassonografia pélvica e ressonância magnética cerebral estrutural) a adolescentes de sexo feminino de 14 anos de idade.
Resultados: Foram incluídas no estudo quatro adolescentes maturadoras precoces (grupo 1) e três maturadoras médias (grupo 2), A idade de início da puberdade foi a única variável biológica que distinguiu os 2 grupos (p=0,05). Apesar de não se encontrar significado estatístico, verificou-se que as maturadoras precoces, apresentavam volumes de todas as estruturas subcorticais estudadas superiores às maturadoras médias, com exceção do núcleo accumbens.
Conclusões: Com base na pesquisa bibliográfica efetuada, este estudo foi o primeiro a tentar relacionar os ritmos de maturação pubertária e o desenvolvimento cerebral através da ressonância magnética. A tendência observada para o aumento do volume das estruturas subcorticais poderá estar relacionado com um possível atraso na maturação do núcleo accumbens nas maturadoras precoces e poderá explicar o aumento da vulnerabilidade deste grupo para comportamentos de risco. Consideramos que poderá constituir o ponto de partida para investigações futuras, de forma a compreender melhor o desenvolvimento adolescente e assim permitir encontrar formas mais eficazes de intervenção.
Palavras-chave: Adolescência; desenvolvimento cerebral; neuroimagem, puberdade; ressonância magnética
INTRODUCTION
Adolescence is defined by marked physical and psychosocial changes.1-6 It is a period of great vulnerability, particularly associated to experimentation and risk behaviours.7 The transition between childhood and adulthood is not straightforward and it is well known that adolescents with distinct rhythms of pubertal maturation have different psychosocial behaviours.2,8-10 Three distinct theories try to explain such differences: the stressful change hypothesis, the off-time hypothesis and the early- timing hypothesis.9 The first theory predicts that regardless of timing, girls experiencing the pubertal transition will manifest higher levels of emotional disturbance than pre or post-pubertal girls. The off-time hypothesis explains that both early and late maturing girls will manifest more psychological problems than their on-time age-mates. Finally, the early-time hypothesis advocates that girls maturing earlier than their peers will be less emotionally stable and will have more behaviour problems. Indeed, several studies have shown that early-maturer girls have a lower self-esteem, a higher risk of drug use and misuse and tend to carry a psychopathological profile, being more vulnerable to peer pressure.3,5,6
Although influenced by numerous factors such as genetics, nutritionandethnicity, whichmayinfluencephysicaldevelopment, puberty is essentially a hormonal and neuroendocrinological process.1,4,7,11,12 Among girls, puberty starts between eight and 13 years of age (mean age: 10 years) with the onset of the thelarche.2,5,7,13,14
Notably, adolescence is recognized as the largest and most dynamic period of neuronal development. Longitudinal studies have revealed that grey matter maturation is represented by an inverted U-shape curve during childhood and adolescence, occurring from posterior to anterior regions of the brain. Accordingly, the prefrontal cortex (PFC), which participates in risk-benefit assessment and decision-making, is described as the last brain region to mature.6,13,15-19 As a result, the immature motivational circuit – limbic system/PFC – favours activity in brain emotional areas, including the nucleus accumbens and the amygdala.4,13,15 These data suggest that there may be a brain developmental explanation for the impulsivity and risk behaviours of adolescence.8
Magnetic resonance imaging (MRI) has been invaluable in the study of the central nervous system in humans in vivo, since it is an innocuous, reproducible and repeatable technique that offers high tissue contrast and spatial resolution.18-20
The purpose of this study was to investigate with MRI the brain development of female adolescents with the same chronological age but distinct rhythms of pubertal maturation.
To avoid potential confounding variables (such sex or endocrinology pathology) only healthy female adolescents were selected.
METHODS
Sample
The sample was identified and recruited from the Outpatient Clinic of the Pediatric Endocrinology Unit of Centro Hospitalar
São João - EPE, Porto, Portugal. All the volunteers were previously followed in this unit by suspected (not confirmed) pathology.
The inclusion criteria were the following: adolescent females of 14 years of age who had the thelarche between eight and nine years of age (early-maturers) or between 10 and 11 years of age (average-maturers). Exclusion criteria included having an associated pathology and / or being under pharmacological therapy. The study was approved by the Ethics Committee of the Centro Hospitalar São João - EPE. All volunteers and their legal representatives signed an informed consent according to the Helsinki Declaration.21
Clinical data
The following data was collected from the individual clinical files: weight, height, body mass index (BMI), the age at thelarche andmenarche, sexualmaturityrating(Tannerstage)(TS), hormonal profile previously performed within three months (including FSH – follicle-stimulating hormone; LH – luteinizing hormone; estradiol; 17OHP – 17 – hydroxyprogesterone; testosterone; DHEAS – dehydroepiandrosterone sulphate; androstenedione; IGF1 – insulin-like growth factor 1 (somatomedin C); FT4 – free thyroxine; TSH – thyroid stimulating hormone), bone age and pelvic ultrasound (including ovary volume, uterine volume and presence of endocavitary line).
Magnetic resonance imaging and brain volumetry
MRI brain examinations were conducted in the Department of Radiology of the Centro Hospitalar São João, EPE, using a 3.0T system (MAGNETOM® Tim Trio, Siemens Medical Solutions, Erlangen, Germany), equipped with a 12-channel head phased- array coil. All the datasets were acquired in a single session, with the presence of a neuroradiologyst. A 3D-T1-w pulse sequence (magnetisation prepared rapid acquisition gradient echo – MPRAGE) was acquired with the following parameters: 160 slices, 265 mm field-of-view (FOV); spatial resolution 1x1x1mm3, TR/TE/f.a. = 2030/2.96ms/9º.
Image processing was performed using the FMRIB Software Library (FSL) analysis tools.22,24 Brain volumetry (total brain, white matter and grey matter volumes) were calculated with the Sienax package from FSL, while the volume of subcortical structures (hippocampus, amygdala, thalamus, caudate nucleus, putamen, globus pallidus and nucleus accumbens) were measured with the FIRST toolbox, also part of FSL.22,26,27
Statistical analysis
Frequency distributions were generated for every categorical variable for the descriptive statistics. For non-homogenous variances, the Mann-Whitney test was adopted. Relations between hormone levels and subcortical structures were assessed using Spearman correlation coefficients. Statistical analyses were performed using SPSS 21 (IBM SPSS software). Results of the brain volumetry and of the volume of subcortical structures are presented as median and standard deviation (SD). Statistical significance was considered for p≤0.05.
RESULTS
Thirteen adolescents were identified. Four were excluded due to no parental authorization (n=3) and participation refusal (n=1). From the nine volunteers that were included in the study, five were early-maturers (group 1) and four were average-maturers (group 2). The demographic characteristics of the sample are detailed in Table1.
The mean age of onset of puberty was 8,75 years for the early-maturers (range 8-11y) and 10,3 years for the average- maturers (range 10-11y), respectively. Menarche was at mean age of 11y for group 1 (minimum 11 years, maximum 12 years) and 12y for group 2 (minimum 11 years, maximum 13 years). All adolescents were TS 5, except for volunteer 5 who was stage 4. There were no differences between groups regarding age at menarche (p = 0,229), weight (p = 0,857) and BMI (p = 0,857).
Pelvic ultrasound evaluation was compatible with the respective pubertal development in all cases. Bone age was higher than chronological age in all adolescents (mean 15.4 ± 0.73 y). The hormonal profile was obtained for eight of the total of nine adolescents (Table 2). Hormonal results of all adolescents were within the reference values for age and gender, except for volunteer 1, 4 and 5, highlighted in Table 1, which were slightly above the reference values but without pathological relevance. Group 1 had higher levels than group 2 (not statistically significant) for FSH (p = 0,400), FT4 (p = 0,400), androstenedione (p = 1) and estradiol (p = 1); and lower levels of LH (p = 0,400), testosterone (p = 0,400), DHEAS (p = 0,400), IgF1 (p = 1), 17OHP (p = 0,700) and TSH (p = 0,629).
Regarding brain MRI, one adolescent did not complete the brain scan (V9). A diencephalic lesion was incidentally detected in another volunteer (V3), which was diagnosed later as a pilocytic astrocytoma. Threrefore both volunteer (V3 and V9) were excluded.
Global brain and subcortical structures volumetry results are presented in Table 2. There were no statistical differences between the two groups for total brain volume, as well as for grey matter and white matter volumes (Figure 1). Concerning the volume of the subcortical structures, group 1 evidenced higher volumes for all subcortical structures (left and right-sided) than group 2, except for the nucleus accumbens (Figures 2 and 3).
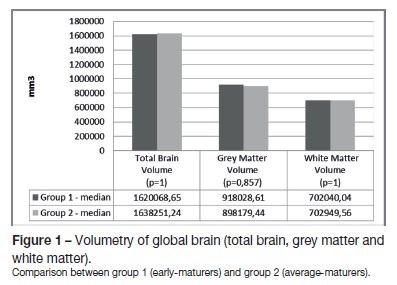
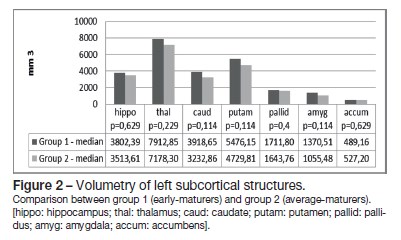
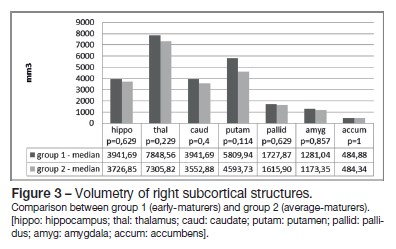
Correlation of the subcortical brain structures volume and hormone levels was performed (Table 3). We found a positive correlation between FSH levels and the volume of the putamina (r = 0,886), and as well as between DHEAS levels and the volume of the left nucleus accumbens (r = 0,829). Negative correlations were found between LH levels and the left (r = -0,943) and right hippocampi volume (r = -1); between testosterone levels and the left caudate nucleus (r = -0,829), left and right putamina (r = -0,829) and globus pallidus (left r = -0,943; right r = -0,886); between estradiol and the left nucleus accumbens (r = -0,886); and between androstenedione levels and the right nucleus accumbens volume (r = -0,943).
DISCUSSION
Our research aimed at assessing brain neurodevelopment by using brain MRI on a clinical sample of adolescents with distinct maturing rhythms. To our knowledge, this study is among the first to address the relation of pubertal maturation timing and the central nervous system development using MRI.
Regarding total brain volume, there were no differences between the early and average-maturers, despite early-maturers had had higher volumes for all subcortical structures except for the nucleus accumbens. It is well known that this nucleus is involved in the connection between the limbic system and the PFC, and is associated with pleasure and reward.
(28) The observed tendency for the higher volumes of all other subcortical structures among early-maturers may be explained by a possible delayed development of the nucleus accubens in this group and this could potentially translate into the increased vulnerability and engagement in risk behaviours typical of early-maturers.
Regarding the hormonal profile, we found that group 1 had higher levels of androstenedione and lower levels of testosterone and DHEAS than group 2, despite not reaching statistical significance. Although no causal linearity has been established between the hormonal profiles and behaviour so far, it has been shown that boys and girls with maladaptive behaviours have higher levels of androstenedione and lower levels of testosterone and DHEAS during their early and middle adolescence, what is in line with the tendency of our results. Furthermore, it is well known the relevant role of the gonadal hormones on the central nervous system maturation, with for example both progesterone and estradiol facilitating myelination in adolescent females, while testosterone has a similar role in males.4,7 It is known as well that the hippocampus and the amygdala have a higher number of estrogen and androgen receptors, respectively, what may explain the largest volume of the amygdala among males during adolescence and hippocampal volume only among females.17,18
While the association between the levels of circulating gonadal hormones and sex-specific differences is well established, the effects of sexual hormones and pubertal stage on brain development, is not yet well understood. Studies are not consensual, with some suggesting a pubertal-related maturation of the hippocampus, amygdala and cortical grey-matter, and others not having been able to find a significant association between sexual hormones and the subcortical structures.12,29,30
The strong correlations between hormones with direct influence on the sexual development (FSH, LH, estradiol, testosterone, DHEAS, androstenedione) and the brain subcortical structures found in our study should be further explored.
The most important limitation of our study was the small sample size, which was related to difficulties in recruiting voluntary adolescents. The accidental finding of a diencephalic tumour in an asymptomatic volunteer, allowed timely surgery, with complete resection.
In spite of the small sample, our results support the hypothesis that brain maturation of early-maturers is not proportionate to biological maturation, in particular to the nucleus accumbens. This study should be seen as a starting point for future research for better understanding adolescents behavior and allowing the design of more efficacious interventions.
ACKNOWLEDGEMENTS
Professor Isabel Ramos, Director of the Department of Radiology of Centro Hospitalar São João - EPE, and of the Group of Medical Imaging and Signal Processing of the Faculty of Medicine of Porto University, Portugal.
REFERÊNCIAS BIBLIOGRÁFICAS
1. Bordini B, Rosenfield RL. Normal pubertal development: Part I: The endocrine basis of puberty. Pediatr Rev 2011; 32: 223-9. [ Links ]
2. Neinstein L. Adolescent health care – A pratical guide. 5th edition. Lippincott Williams and Wilkins. Philadelphia. 2008, pp 3-31. [ Links ]
3. Patton GC, Viner R. Pubertal transitions in Health. Lancet 2007; 369: 1130-7. [ Links ]
4. Vigil P, Orellana RF, Cortes ME, Molina CT, Switzer BE, Klaus H. Endocrine modulation of the brain: A review. J Pediatr Adoles Gynecol 2011; 24(6): 330-7. [ Links ]
5. Rosenfield R. Normal Pubertal Development: Part II: Clinical aspects of puberty. Pediatr Rev 2011; 32: 281-91. [ Links ]
6. Hazen E, Schlozman S, Beresin E. Adolescent Psychological Development: A review. Pediatr Rev 2008; 29: 161-8. [ Links ]
7. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24: 417-63. [ Links ]
8. Romer D. Adolescent risk taking, impulsivity and brain development: Implications for prevention. Dev Psychobiol 2010; 52: 263-76. [ Links ]
9. Ge X, Conger RD, Jr Glen HE. Coming of age too early: pubertal influences on girls` vulnerability to psychological distress. Child Dev 1996; 67: 3386-400. [ Links ]
10. Graber JA, Levinsohn PM, Seeley JR, Brooks-Gunn J. Is Psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry 1997; 36:1768-76. [ Links ]
11. Sisk CL, Zehr JL. Pubertal Hormones organize the adolescent brain and behavior. Front Neuroendocrinol 2005; 26: 163-74. [ Links ]
12. Neufang S, Specht K, Hausmann M, Gurturkun O, Herpertez-Dahlmann B, Fink G et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 2009; 19: 464-73. [ Links ]
13. Blakmore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp 2010; 31: 926-33. [ Links ]
14. Kaplowitz PB. Delayed Puberty. Pediatric Rev 2010; 31: 189-95. [ Links ]
15. Jonhson SB, Blum R, Giedd J. Adolescent Maturity and the Brain: The promise and Pitfalls of Neuroscience Research in Adolescent Health Policy. J Adolesc Health 2009; 45: 216- 21. [ Links ]
16. Blakemore SJ. The social brain in adolescence. Nature Rev Neurosci 2008; 9: 267-77. [ Links ]
17. Giedd JN. The Teen Brain: Insights from Neuroimaging. J Adolesc Health 2008; 42: 335-343. [ Links ]
18. Giedd JN, Stockman M, Weddle C, Liverpool M, Alexander- Bloch A, Wallace GL, et al. Anatomic magnetic resonance imaging of developing and adolescent brain and effects of genetic variation. Neuropsychol Rev 2010; 20: 349-61. [ Links ]
19. Luna B, Padmanabhan A, OHearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 2010; 72: 101-13. [ Links ]
20. Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev 2011; 35: 1654-64. [ Links ]
21. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. October 2013. [Cited 2014 november 11]. Available from URL: www.wma.net/en/30publications/10policies/b3/ . [ Links ]
22. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62: 782-90. [ Links ]
23. Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009; 45: S173-86. [ Links ]
24. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: 208-19. [ Links ]
25. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 7: 43-55. [ Links ]
26. Smith SM, De Stefano N, Jenkinson M, Matthews P.M. Normalised accurate measurement of longitudinal brain change. J Comput Assist Tomogr 2001; 25: 466-75. [ Links ]
27. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011; 56: 907-22. [ Links ]
28. Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: Assessing pleasure and arousal in the brains reward circuitry. Hum Brain Mapp 2010; 31: 1446–57. [ Links ]
29. Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex 2011; 21: 636-46. [ Links ]
30. Koolschijn PCMP, Peper JS, Crone EA. The Influence of Sex Steroids on Structural Brain Maturation in Adolescence. Plos One 2014; 9: e83929. [ Links ]
CORRESPONDENCE TO
Paula Fonseca
Department of Pediatrics
Unidade de Famalicão
Centro Hospitalar Médio Ave
Rua Cupertino de Miranda, Apartado 31, 4761-917 Vila Nova de Famalicão
Email: paulamrfonseca@hotmail.com
Received for publication: 04.04.2016 Accepted in revised form: 13.10.2016














