INTRODUCTION
Fluid management is a key component of end-stage renal disease (ESRD) treatment. Sustained extra-cellular fluid (ECF) overload increases the risk of cardiovascular events and associates with a higher prevalence of arterial hypertension, left ventricular hypertrophy, dilated cardiomyopathy1 and higher morbidity and mortality.2 The physiologic nature of peritoneal dialysis (PD) allows for gentle ultrafiltration during all hours, avoiding many of the complications inherent to a thriceweekly in-center hemodialysis (HD) program, like excessive interdialytic weight gain and intradialytic hypotension.2,3 Volume overload was once considered to occur more frequently in patients on PD than those on HD, a difference attributed to PD’s lower efficiency of volume removal.2 Since then, clinical practices have evolved for both HD and PD and it is now widely accepted that the volume status is more dependent of dialysis prescription quality and patient’ characteristics than the type of dialysis technique.3 Furthermore, some evidence suggests that the extracellular water/total body water ratio - assessed by multiple frequency bioelectrical impedance analysis (MF-BIA) - appears to be similar between PD patients and HD patients before treatment.3
The International Society of Peritoneal Dialysis (ISPD) Adult Cardiovascular and Metabolic Guidelines, released in 2015, recommended that hydration status should be clinically assessed on a regular basis during every follow-up visit and more often if indicated.4 In the 2020 ISPD’s high-quality PD prescription guideline update, the workgroup reviewed the limitations of using small solute clearance (Kt/V) as a treatment target in PD prescription. A new approach to PD adequacy, incorporating dimensions such as volume management (which encompasses both salt and fluid removal) and blood pressure (BP) control, was recommended in assessing the quality of PD prescription.5 Optimal fluid balance in PD requires both urinary and peritoneal water and sodium removal and restriction of dietary sodium intake (<2 g/day).
Patient’s adherence to a low-salt diet is difficult to evaluate, since the measurement and monitoring of dietary sodium consumption is unstandardized. Therefore, it is most frequently assessed by indirect measures, like fluid status and BP control. Recently, Kim et al conducted a study in PD patients comparing renal and peritoneal sodium removal and sodium intake by dietary recollection. In their study total sodium removal had a strong positive correlation (r=0.6) with sodium intake, suggesting that the measurement of total sodium removal during the assessment of dialysis adequacy could be an effective and simple method to estimate dietary sodium intake in PD patients.6 This correlation was translated mathematically into different formulas for both patients with and without residual diuresis, allowing for an estimation of sodium intake through peritoneal and urine sodium removal.
We aim to study the relationship between estimated dietary sodium intake (according to the formulas presented in Kim SM’s work) and blood pressure control, as well as medication burden in PD patients.
METHODS
We conducted an observational, cross-sectional study in chronic PD patients enrolled in our Dialysis Unit in March 2022. Patients were included only if they had at least six months of dialysis vintage. Patients were excluded if there was any evidence of current systemic (peritonitis, bacterial pneumonia, cellulitis) or local (tunnel or exit-site) infections.
Demographic data (such as gender, race and age), prior diseases (namely diabetes, hypertension, dyslipidaemia, obesity and cardiovascular disease) were collected retrospectively from the existing patient records.
For each patient, the number of anti-hypertension drugs, ambulatory BP measurements from the previous three months, urinary output and PD-related information (transporter type, mean technique Kt/V from the previous three months) were collected at the time of the last 24-hour urine and peritoneal fluid collection. BP control was categorized according to the European Society of Cardiology guidelines in: controlled- mean systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg; stage one- mean SBP 140-160 mmHg and/or DBP 90-100 mmHg; stage 2- SBP >160 mmHg and/or DBP >100 mmHg; stage 3- mean SBP >180 mmHg and/or DBP 110 mmHg. Sodium intake estimation equations (mg/day), according to Kim SM et al, differed in patients with and without residual renal function (RRF): RRF (+): (15.4 × total sodium removal (mEq/d)) + 609. RRF (-): (19.3 × peritoneal sodium removal (mEq/d)) + 211.
Peritoneal and urinary sodium removal was measured according to urinary and peritoneal sodium concentration from 24-hour collections, respectively. To assess patient’s perception of sodium consumption, we also performed a qualitative evaluation of sodium intake by asking patients if they were compliant or non-compliant with a low-sodium diet (intake <2 g).
Statistical analysis was performed using IBM-SPSS Statistics v22 and the confidence interval was set on 95%. A p value < 0.05 was considered statistically significant. The sample will be described globally and by groups in terms of the distribution of the descriptive variables by summary statistics depending on the type of variable and its distribution. Categorical variables will be described as relative frequency (absolute frequency). Numerical continuous and discrete data will be described as mean ± standard deviation for normal distributed variables and median (interquartile range) for non-normal distributed variables. Comparison of means and frequencies of normally distributed variables were calculated using t-tests and the χ2 test. Pearson’s correlation was used to identify a correlation between different variables.
Independent samples t-test was used to compare demographic data, prior diseases, ambulatory BP measurements and estimated sodium intake. The receiver operating characteristic (ROC) curves and Youden index were used to identify a cut off point for estimated dietary sodium intake and uncontrolled BP. On a second analysis, sodium consumption was compared with average blood pressure, number of blood pressure drugs taken and urinary output using independente samples t-test. Afterwards, patient’ perception of sodium consumption was also compared with calculated sodium intake.
RESULTS
Our sample was composed of 82 PD patients (96.5% of our population): most were Caucasian (92.7%, n=76), males (58.5%, n=48) and had a mean age of 54.1±14.7 years. Chronic kidney disease of undetermined etiology was the most prevalent cause of ESRD (18.3%, n=15), followed by hypertensive nephropathy (15.9%, n=13), diabetic nephropathy (14.6%, n=12) and IgA nephropathy (12.2%, n=10). Our sample presented with significant identifiable cardiovascular (CV) risk factors: arterial hypertension (100%, n=82), dyslipidemia (64.6%, n=53), obesity (32.9%, n=27), diabetes (24.4%, n=20) and heart failure (23.2%, n=19). Consequently, 28% (n=23) were categorized as having a very-high CV risk, according to ESC’s CV risk classification. Regarding the PD technique, most patients were on continuous ambulatory PD (CAPD) (63.4%, n=52) and had a PD vintage of 26.2±18.7 months. Peritoneal membrane transport analysis through Peritoneal Equilibration Test (PET) revealed a high prevalence of average-high transporters (69.5%, n=57), followed by low-average (19.5%, n=16) and high (11%, n=9) transporters. Residual diuresis was present in 85.4% (n=70), with a mean value of 1257±867 mL/day. In respect to PD adequacy parameters, our sample presented with adequate dialysis efficiency: 96.3% (n=79) had a weekly Kt/V (mean from the previous three months) ≥ 1.7, which was reflected in a mean weekly Kt/V of 2.2±0.4, and 51.2% (n=42) had controlled BP. The remaining patients had stage one (23.2%, n=19) and stage 2 (25.6%, n=21) hypertension.
In this regard systolic blood pressure (SBP) averaged 134.7±15.6 mmHg, diastolic blood pressure (DBP) 82.5±12.9 mmHg and mean arterial pressure (MAP) 98.9±12.8 mmHg. The prevalence of BP control was independent of age, gender, race and PD-related parameters (PD technique, mean weekly Kt/V, residual diuresis).
Across our series, each patient was treated with an average 4.0±1.6 drugs for BP control. Males (4.4±1.5 vs 3.5±1.5, p=0.01) and uncontrolled BP patients (4.8±1.4 vs 3.3±1.5, p<0.001) had a higher medication burden. Age, dialysis vintage and PD-related parameters did not influence the number of prescribed drugs.
In patients with residual diuresis, mean renal and peritoneal sodium clearance were 106.6±60.6 mmol/day and 85.9±64.4 mmol/day, respectively; those without residual diuresis had higher peritoneal sodium clearance (158.5±65.0 mmol/day). Higher SBP (Pearson’s correlation coefficient (r)=0.45, p<0.0001) and higher residual diuresis (r=0.63, p<0.0001) associated with higher urinary sodium removal.
Compliance to a low-sodium diet (<2 g), calculated according to urinary and peritoneal sodium removal, was achieved in 8.5% (n=7).
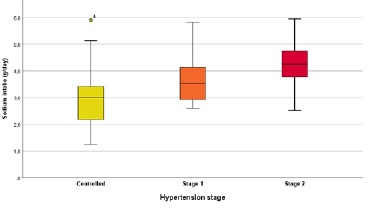
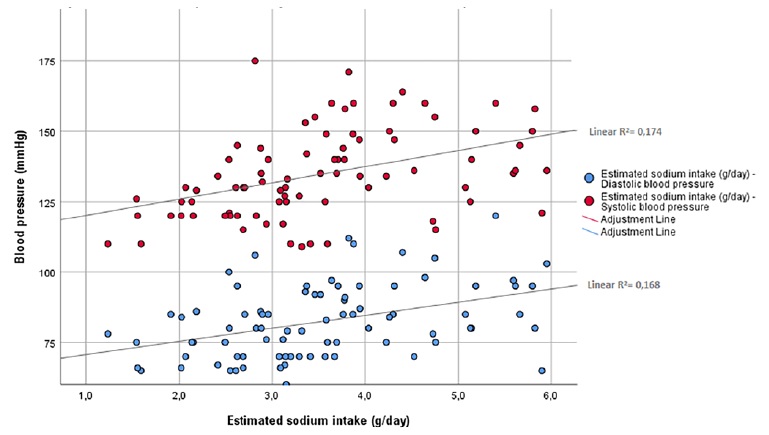
Mean estimated dietary sodium intake was 3.5±1.1 g and was higher in males (3.8±1.3 vs 3.1±0.8 g, p=0.007), CAPD patients (3.8±1.2 vs 3.0±0.9 g, p=0.005) and patients with uncontrolled hypertension (4.0±1.0 vs 3.0±1.0 g, p<0.0001), with higher consumptions with increasing hypertension’ class severity (Fig. 1). Interestingly, patients with low-income had also higher calculated sodium consumptions (4.2±1.1 vs 2.7±0.9 g, p<0.0001) when compared to patients with normal or high incomes. A strong correlation was found between estimated dietary sodium intake and SBP (r=0.50, p<0.0001), DBP (r=0.43, p<0.0001) and the number of prescribed anti-hypertensive drugs (r=0.53, p<0.0001). Fig. 2 shows the distribution of SBP and DBP according to estimated sodium intake. Sodium intake also correlated with residual diuresis (r= 0.4, p<0.0001) and with total urinary sodium removal (r=0.53, p<0.0001).
Salt intake did not correlate with age, dialysis vintage and was similar across different ethnic groups and in patients with identified CV risk factors (obesity, dyslipidaemia, diabetes, heart failure). In our series, a cut-off value for sodium intake of 3.3 g was found to associate with a higher risk of uncontrolled BP (Area under de curve(AUC):0.78, sensitivity:0.80, specificity:0.74). This sub-population also had a significantly higher mean SBP (142.1±14.9 vs 126.5±11.9 mmHg, p<0.0001), DBP (88.2±12.5 vs 76.1±10.2 mmHg, p<0.0001) and antihypertensive medication burden (4.8±1.4 vs 3.2±1.2, p<0.0001).
A qualitative evaluation of patients’ dietary sodium consumption revealed that 58.5% (n=48) stated compliance to a low-sodium diet, from which 14.6% (n=7) had an estimated sodium intake inferior to two grams per day. Further analysis of the sub-group of patients with higher sodium intake (>3.3 g, n=42) showed that only 2.3% (n=1) admitted non-compliance with a low-sodium diet.
DISCUSSION
Hypertension is highly prevalent amongst peritoneal dialysis patients and increases the risk of cardiovascular disease.7 Although the etiology is often multifactorial, ECF overload is undoubtedly the most important factor.7 Sodium plays a pivotal role in ECF volume regulation: it is the main extracellular cation and is the determinant of intra-vascular volume. Dietary sodium intake contributes to this regulation by increasing the sodium load and stimulating thirst which further contributes to fluid overload.8,9Additionally, sodium cations also accumulate dynamically in glycosaminoglycan networks on the interstitial space.10 This system may function as an important buffer; however, these secluded cations escape renal regulatory function and are more difficult to remove from the body.10,11Interstitial sodium accumulation induces the upregulation of inflammatory mediators via the tonicity-responsive enhancer-binding protein (TonEBP) in some types of cells (cardiomyocyte and peritoneal mesothelial cells), which ultimately leads to tissue fibrosis and dysfunction and associates with high morbility and mortality.12
Despite the ISPD recommendations for PD patients to restrict dietary sodium consumption, compliance is often challenging. Amalia RI et al conducted a semi-quantitative study in PD patients for sodium intake estimation through dietary records. Whereas median sodium intake was 2.4 g (1.64-3.34), most patients were found to be consuming more dietary sodium than recommended.8 In a more recent study, Gong N et al had similar results: sodium intake averaged 2.1 ± 1.4 g and compliance to a low-salt diet was achieved in only 50%, highlighting the difficulty of dietary salt restriction in PD patients.9
In our series, compliance to a low-sodium diet (<2 g) was achieved in only 8.5%. Estimated sodium intake averaged 3.5 g/day and was higher amongst patients with low-income and those with more severe hypertension. Also, a cut-off value for sodium intake of 3.3 g was found to predict a higher risk of uncontrolled hypertension. These results differed from previous studies both in patient compliance and estimated dietary sodium intake. This difference could be due to samples’ asymmetry regarding socioeconomic characteristics: our sample was mainly composed by patients that live in rural areas (37.8%, n=31) and/or have lower mean income values (28.1%, n=23), which may directly impact their power of choice when shopping and may lead to poorer food’ nutritional quality. Patient’s unawareness of the sodium content of most commercial foods and difficulty to change dietary habits could have also contributed to the registered poor patient compliance.
We found a significant correlation between estimated sodium intake and SBP, DBP and anti-hypertensive medication burden (p<0.001). In addition, we registered a high anti-hypertensive medication burden across our sample, as each patient was treated with na average four drugs per day. Most importantly, there was a striking difference between patients’ perception of sodium consumption and the calculated sodium intake, especially in patients with higher sodium consumption (>3.3 g). These results underline the importance of a low-sodium diet in BP control and suggest that, sometimes, as clinicians, our first approach to uncontrolled hypertension is to increase the dosage of an already prescribed medication and/or add a new drug, when we should focus on dietary counselling and improve renal sodium removal. Also, an accurate and individualized PD prescription, supported by peritoneal membrane transport characteristics, is na effective tool to maximize sodium removal and control volume status. Furthermore, a strong positive correlation between estimated dietary sodium consumption, SBP, urinary sodium excretion and residual diuresis was found. This association, explained by a phenomenon called pressure natriuresis, is an important physiologic adaptive mechanism in which higher renal arterial perfusion pressure causes an increase in urinary sodium excretion.
Our study was limited by the formulas used to estimate sodium intake in our sample. As these formulas originated from a study including only Korean PD patients, they may not correctly translate to Portuguese PD patients. However, as recent studies suggested, Korean and Portuguese population have similar average daily sodium intakes (Korea 4.29 g and Portugal 4.28 g),13,14which may translate to similar equations. Our study also failed to include MF-BIA measurements, which better correlate with patient’ fluid status, especially when compared to BP alone. However, this material was not available in our PD Unit at the time of study design and consequently could not be included.
Another limitation may arise from the retrospective nature of the data collected as some information may be lacking in patient records. Our results highlight the importance of dietary salt restriction in BP control and reinforce the need for a dietary consultation to educate our patients regarding available low and free-sodium foods. Although dietary counselling alone is not sufficient to achieve BP control, it remains a very effective adjunctive therapy, especially when done by specialized staff.
Future studies should focus on implementing a new, less restrictive target for dietary sodium intake in PD patients as it could improve patient compliance by providing a more feasible goal.