INTRODUCTION
Successful kidney transplantation (KT) is the treatment of choice for end-stage kidney disease (ESKD), as it affords not only superior survival, but also better quality of life for a lower cost when compared to dialysis treatments.1-3 Such benefits are maintained even in higher-risk groups, such as the elderly and the diabetic patients.2,4,5
As such, the option of a kidney transplant (KT) should be discussed with all eligible patients with chronic kidney disease (CKD) expected to reach ESKD.5
Despite its many benefits, patients may suffer complications which compromise the success rate of KT. There is particular concern regarding the development of delayed graft function (DGF), defined as the need for dialysis in the first postoperative week, which has been associated with both worse short-term and long-term outcomes and higher rejection rates.6-10
Inflammation plays a key role in the development of DGF. The leading cause for the development of DGF is ischemia-reperfusion injury, which is responsible for increasing inflammation in the graft tissue, having a considerable impact on KT outcomes.11-13 Neutrophils play a key role in the development of ischemia-reperfusion injury after transplant as, upon infiltrating the ischemic tissue and releasing toxic metabolites, lead to tissue damage and increased tubular and capillary leakage.14-17 The key role inflammation plays in patient outcomes is further highlighted by the importance immunosuppression plays in the management of KT recipients.18,19
Several studies have shown that the damage caused by ischemiareperfusion injury is considerably more severe with prolonged cold and warm ischemia times.12,20-22 Alongside inflammation, many other factors have been associated with the risk of developing DGF, such as the type of donor, panel reactive antibody (PRA) and human leukocyte antigen (HLA) mismatches, among others.23,24
The neutrophils to lymphocytes and platelets (NLP) ratio constitutes an affordable biomarker of systemic inflammation and can be easily determined from a complete blood cell count.25,26 Studies previously demonstrated the utility of the neutrophil-lymphocyte (NL) ratio in predicting postoperative outcome, progression of CKD and cardiovascular mortality.26-28 It has also been studied as an early predictor of acute kidney injury (AKI) in many settings and by adding the platelet count to this ratio the predictive ability of AKI after cardiovascular and abdominal surgery was increased.29,30
We aimed to assess whether the NLP ratio may be used as an early predictor of DGF in KT patients.
METHODS
This is a retrospective cohort of patients submitted to kidney transplantation at the Centro Hospitalar Universitário Lisboa Norte (CHULN), in Lisbon, Portugal, between 1 January 2010 and 31 December 2020. This study was approved by the Ethical Committee, in agreement with institutional guidelines. Informed consente was waived, given the retrospective and non-interventional nature of the study.
We selected as eligible all adult patients submitted to deceased donor renal transplantation in our centre during the study period. No exclusion criteria were utilized.
According to our department protocol, we use Thymoglobulin® in patients at high risk of rejection, which, in our unit, corresponds to patients with a PRA >10% or DSAs, third KT or second KT in melanodermic patients. In the remaining patients, we use basiliximab.
Data was collected from individual electronic clinical records. The following variables were analysed: recipient demographic characteristics (age at start of renal replacement therapy and age at KT, gender, race); comorbidities (hypertension, diabetes mellitus, obesity); histocompatibility according to virtual PRA and HLA mismatches; donor characteristics (age and type of donor); procedure parameters (cold and warm ischemia time); and induction immunosuppression. Laboratory values before transplant, 24 hours after transplantation, and at discharge were collected and comprised serum hemoglobin, NL ratio, platelet count and serum creatinine. The NLP ratio was calculated as the neutrophil count multiplied by one hundred and then divided by the product of the lymphocyte and platelet counts.
The analysed outcome was occurrence of DGF. Delayed graft function was defined as the need for at least one dialysis treatment within the first week after transplantation or failure of the serum creatinine to decline by more than 25 percent within 24 hours of transplant surgery.
Categorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean ± standard deviation. Continuous variables were compared with the Student’s t-test and categorical variables were compared with the Chi-square test. All variables underwent univariate analysis to determine statistically significant factors which may have contributed to DGF. Subsequently, variables with a significant statistical difference were included in the multivariate analysis using the logistic regression method. Data were expressed as odds ratios (OR) with 95% confidence intervals (CI). Statistical significance was defined as a P-value <0.05. Statistical analysis was performed with the statistical software package SPSS for windows (version 21.0).
RESULTS
During the study period, a total of 527 patient underwent kidney transplantation at CHULN. Patients’ characteristics are presented in Table 1. Mean age at kidney transplantation was 49.8 ± 12.9 years and more than half of patients were male (n=308, 58.4%). Patients had been under renal replacement therapy for 5.9 ± 4 years before transplantation. Most patients had arterial hypertension (n=364, 71.9%), 8.7% were diabetic (n=44) and 15.1% were obese (n=79). Mean PRA value was 12.4% ± 22.8%. Approximately half of the patients had more than three HLA mismatches (n=215, 55.4%). Donors were 52.8 ± 14.4 years old and in almost half of the patients (n=252, 47.8%) expanded criteria donors (ECD) were used.
Table 1 Baseline characteristics and according to delayed graft function
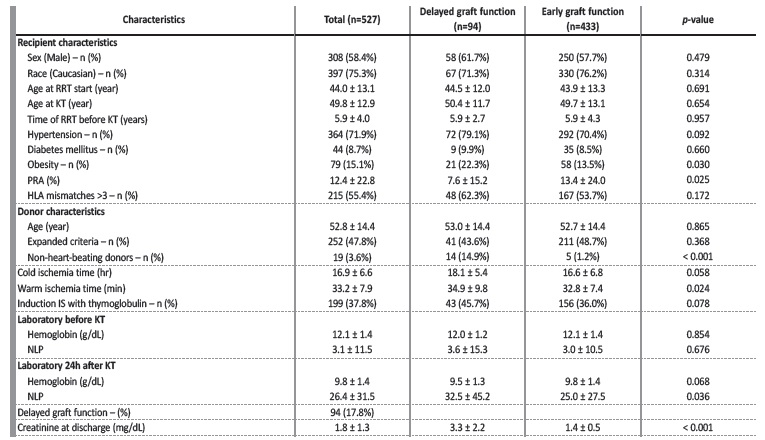
RRT - renal replacement therapy; KT - kidney transplant; PRA - panel reactive antibodies; HLA - human leukocyte antigen; IS - immunosuppression; NLP - neutrophils to lymphocytes and platelets ratio
Donation after circulatory determination of death (non-heart-beating donors) was present in nineteen (3.6%) donors. Cold ischemia time (CIT) was 16.9 ± 6.6 hours and warm ischemia time (WIT) 33.2 ± 7.9 minutes. Rabbit anti-thymocyte immunoglobulin (Thymoglobulin ®) was the immunosuppressant of choice for induction in 199 patients (37.8%).
Concerning laboratory results prior to KT, mean haemoglobin was 12.1 ± 1.4 g/dL and mean NLP was 3.1 ± 1.5, and at 24 hours after KT mean haemoglobin was 9.8 ± 1.4 g/dL and mean NLP was 26.4 ± 31.5.
Delayed graft function
Ninety-four patients (17.8%) had DGF.
No differences were found regarding the presence of other comorbidities, namely hypertension and diabetes. Gender, age at the start of renal replacement therapy and at KT were similar between populations.
Donor age was also similar. No differences were noted regarding HLA mismatches, laboratory parameters at admission or other characteristics, namely regarding the use of rabbit anti-thymocyte immunoglobulin as induction therapy.
These patients were more likely to be obese (22.3% vs 13.5%, p=0.030; unadjusted OR 1.845, 95% CI 1.055, 3.226, p=0.032) and had lower PRA (7.6 ± 15.2 vs 13.4 ± 24.0, p=0.025; unadjusted OR 0.986, 95% CI 0.973, 0.999, p=0.029). They received more grafts from non-heart-beating donors (14.9% vs 1.2%, p<0.001; unadjusted OR 14.980, 95% CI 5.249, 42.750, p<0.001) and had a longer WIT (34.9 ±
9.8 vs 32.8 ± 7.4 minutes, p=0.024; unadjusted OR 1.030, 95% CI 1.003, 1.057, p=0.027). NLP ratio 24 hours after KT was greater among patients who had DGF (32.5 ± 45,2 vs 25.0 ± 27.5, p=0.036; unadjusted OR 1.006, 95% CI 1.000, 1.012, p=0.044).
Post-KT NLP was higher in patients submitted to induction therapy with lymphocyte depleting antibodies (50.2 ± 40.3 vs 11.9 ± 7.4 in patients treated with basiliximab, p<0.001), but it was found to be higher even before KT (5.2 ± 1.8 in patients treated with rabbit antithymocyte immunoglobulin versus 1.9 ± 1.2 in patients treated with basiliximab, p=0.001), and thereby before induction therapy, and not predictive of DGF (Fig. 1).
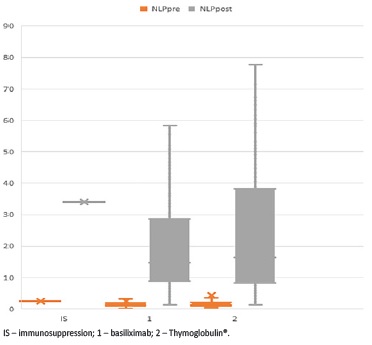
Figure 1 Histograms showing de NLP ratio before and after transplantation according to the induction therapy used
As expected, creatinine at discharge (3.3 ± 2.2 vs 1.4 ± 0.5, p<0.001) was higher in patients with DGF. As presented in Table 2, in a multivariate analysis, grafts from non-heart-beating donors (OR 13.989, 95% CI 4.741, 41.274, p<0.001), longer WIT (OR 1.035, 95% CI 1.007, 1.064, p=0.014) and higher NLP ratio 24 hours after transplantation (OR 1.009, 95% CI 1.002, 1.016, p=0.015) were independently associated with DGF. Additionally, having a lower PRA was associated with higher risk of DGF (OR 0.980, 95% CI 0.966, 0.995, p=0.008).
Table 2 Univariate and Multivariate analysis of factors predictive of DGF
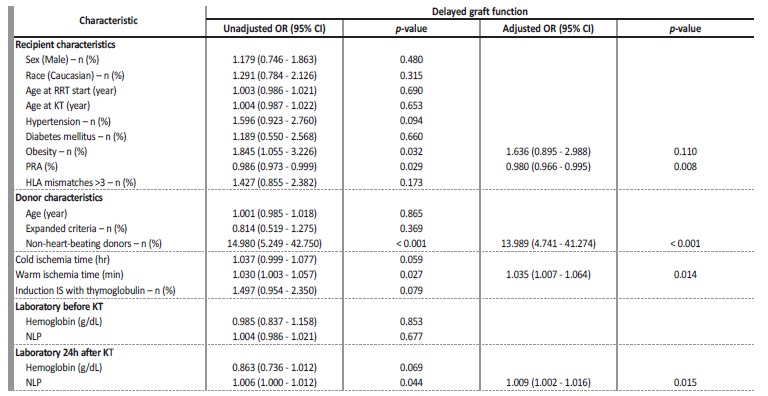
DGF - delayed graft function; RRT - renal replacement therapy; KT - kidney transplant; PRA - panel reactive antibodies; HLA - human leukocyte antigen; IS - Immunosuppression; NLP - neutrophils to lymphocytes and platelets ratio
DISCUSSION
In our cohort, a higher NLP ratio at 24 hours after KT was independently associated with DGF. This reflects the impact of inflammation on KT outcomes and highlights the role of the NLP ratio as a sensitive marker of systemic inflammatory response after KT. A higher NLP was present in high-risk immunological patients (those ultimately presenting criteria for induction therapy with lymphocyte-depleting agents) even before the administration of rabbit anti-thymocyte immunoglobulin, suggesting the presence of a pre-existing pro-inflammatory state. Longer WIT, grafts from non-heart-beating donors and lower PRA were also independently associated with DGF.
Studies conducted in this field have shown that the risk of developing DGF after KT is, on average, between 23% and 31%.7,31,32 However, certain determinants invariably influence the reported incidence, with it ranging approximately between 4% and 10% in living donor transplants and 19% and 70% in deceased donor KT.33,34 Donor type can also influence the incidence of DGF, as shown in a study by Zens et al, in which donation after cardiac death was associated with a higher risk of developing DGF - between 45.1% and 55.3% - when compared to that of donation after brain death, which was between 18.7% and 31.5%.35
In our study, the incidence of DGF was 17.8%, which is lower than what is to be expected when looking at results from other similar studies. Still, at present, DGF represents a common early complication in KT, which is associated with poorer graft outcomes, prolonged post-operative hospitalization, and higher rejection rates. In the worst cases it can even lead to graft failure.36,37 Hence why, in our study, the serum creatinine at discharge was higher among the patients who developed DGF.
Many factors have been associated with the risk of developing DGF, with several studies reporting that older patients, male patients, noncaucasian patients, and patients with a higher body mass index have an increased risk of developing DGF after transplant.10,11,32,34,36 However, of these, only obesity showed any significant risk in our study.
Other commonly cited risk factors include higher PRA and HLA mismatches.23,32,34,38 However, no significant association was shown between the number of HLA mismatches and DGF, and patients who developed DGF seem to have had lower PRA, which contradicts some of the data available in other studies. For this reason, it would be of interest to our study to obtain more information regarding the donorspecific antibodies, since these are not taken into account when looking at the PRA test, rendering it a nonspecific and relatively insensitive test.39,40 Unfortunately, these assays were not widely available throughout the course of our study period and data could not be collected for all patients. A plausible explanation is that we only select patients with low immunological risk for kidneys of non-heart beating donors.
In response to the growing demand for KT, ECD and non-heartbeating donors have been used much more frequently. Although some studies have argued that patients who have received grafts from ECD have a higher risk of developing DGF, others, such as the study conducted by Carter et al, argue that the use of ECD decreases the risk of DGF by decreasing the CIT.34,41,42 However, in our study, no significant association was demonstrated between the use of ECD and the risk of developing DGF. In studies conducted by Irish et al and Zens et al, patients who received grafts from non-heart beating donors had a greater risk of developing DGF, something which coincides with the data we have gathered.35,38
CIT has been strongly associated with DGF in many studies, with a study conducted by Ojo et al highlighting a 23% increase in the risk of DGF for every 6 hours of cold ischemia.23,32,38,43 In our study however, CIT showed no significantly higher risk of developing DGF. This could be due to the fact that CIT were high in both the patients who developed DGF and those who did not. For example, in the study by Melih et al, the mean CIT was around 12 hours, and they found a statistically significant association between longer CIT and DGF.34 Meanwhile, in a study conducted by Halazun et al, an average CIT of over 15 hours was also reported, and no association with DGF was found.16
Yet, WIT did emerge as independently associated with DGF in our study, something which has previously been highlighted as an importante risk factor of DGF alongside CIT.44,45
The main mechanism behind DGF is the ischemia-reperfusion injury. The ischemia is first experienced by renal grafts from the moment they are separated from the donor blood supply. Beginning with a short period of surgical warm ischemia during donor organ extraction, then a lengthy cold ischemic period in hypothermal preserving solution before ending with warm ischemia during implantation in the recipient.13 After revascularization, reperfusion injury takes place, in which blood flow in post-ischemic kidneys will activate a sequence of events that aggravate renal injury. A key factor associated in this is neutrophil infiltration and activation in the ischemic tissue to induce tissue damage.12,13,38
In recent years, the role of platelet and leukocyte interactions has emerged as a critical step in leukocyte recruitment, migration, and activation in inflammation. Immune responses are thus modulated by the interaction between neutrophils, monocytes, lymphocytes and platelets.46
The NL ratio has been described as an indirect marker of inflammation and it has been continuously proven as a useful predictor of the development of AKI.27,29,47,48 The NLP ratio was developed thereafter by adding the platelet count to the NL ratio. Koo et al first described its association with postoperative AKI and mortality after cardiovascular surgery.25 In one study by Gameiro et al, the postoperative NLP ratio after major abdominal surgery was independently associated with AKI.26 In another study by Gameiro et al, the NLP ratio in septic-AKI patients at ICU admission revealed an association with in-hospital mortality.30
It has also been used in other fields, such as in a study conducted by Chae et al, in which it was found that the NLP ratio appeared to be a superior predictor of late mortality in patients with major trauma who underwent emergency surgery when compared with pre-existing trauma scores, as it better reflected the systemic inflammation status.49 Cakir Guney et al also linked the NLP ratio with the risk of ICU admission, in-hospital death and the requirement of mechanical ventilation in patients with COVID-19.50
Considering the role of neutrophils, lymphocytes and platelets in DGF, the NLP ratio could be an interesting marker or early predictor of DGF. Indeed, in this study, the NLP ratio at 24 hours post-transplant was shown to be independently associated with DGF. As expected, given the way it is calculated, post-KT NLP was higher in patients submitted to induction therapy with lymphocyte depleting antibodies, as lymphocyte depletion induced by Thymoglobulin® inevitably increases the NLP ratio.51
However, pre-KT NLP was found to be higher in patients who underwent Thymoglobulin® even before induction therapy. This might be explained by the fact that such patients have been submitted to more sensitization events or that their immunological risk reflects a chronic subclinical inflammatory state, as we use Thymoglobulin® in patients at higher immunological risk. Thereby, pre-KT NLP was not predictive of DGF, irrespective of the immunosuppressant used for induction therapy.
Ours is the first study to report the association between a higher postoperative NLP ratio and DGF. Still, we need to address some limitations. Firstly, although we have an adequate sample size, the results may be compromised by the single centre and retrospective nature of the study. Indeed, classic risk factos for DGF such as age, extended criteria donors and cold ischemia time were not confirmed in this analysis which could reflect uncertain external validity. Secondly, we did not analyse the NLP ratio beyond the first 24 hours after transplant, which could provide predictive value. Thirdly, we did not find a suitable cut--off value of NLP with enough specificity and sensibility for predicting DGF. In addition, although a multivariate analysis was conducted to identify independent predictors, some unmeasured confounders might be present and have affected the results of the study. Finally, we did not have a validation cohort.
Hence the results highlighted in our study require validation by larger multicentre cohorts. However, the data we gathered may still be useful in developing strategies to identify patients at risk of developing DGF.
In this study, grafts from non-heart-beating donors, longer WIT, lower PRA, and higher NLP ratio at 24 hours after transplantation were shown to be independently associated with DGF. It is extremely important to find adequate early predictors of DGF, as it still constitutes a common early complication in KT, which may lead to several adverse short and long-term outcomes, such as increased length of hospital stay and increased risk of acute rejection.
Therefore, the NLP ratio may prove to be a useful tool in the future, given its low cost and easy applicability. Further studies evaluating the use of the NLP ratio as an early predictor of DGF are required to identify at-risk populations, employ preventive strategies, guarantee an early diagnosis, and prompt na adequate treatment, ultimately improving patient outcomes.















