INTRODUCTION
Wunderlich syndrome (WS) is a rare condition characterized by atraumatic spontaneous renal hemorrhage (SRH). Though classically associated to Lenk’s triad (acute flank pain, flank mass and hypovolemic shock1), such presentation is only seen in less than a quarter of patients.2 Clinical manifestations can be varied and unspecific.1
A systematic literature review performed by Ahn et al in 2017 elucidated the contemporary underlying causes of this rare entity.3
Their analysis included all reported cases of Wunderlich syndrome (n = 102) from the year 2000 to 2016 and the main results were similar to a previous meta-analysis performed by Zhang et al in 2002.4 Renal neoplasms were the most common cause (56.9%), of which angiomyolipomas represented the majority of cases (74.1%) and were mainly seen in females. In contrast, malignant neoplasms such as renal cell carcinoma or leiomyosarcoma, were more predominant in males.
Poliarteritis nodosa was the second most common cause of WS (11.8%). In this meta-analysis, ruptured renal cyst was only seen once and 6% of cases underlying cause was not determined. However, patients on dialysis or anticoagulation were excluded from this meta-analysis.3
Early detection is of outmost importance because WS potential for causing hemodynamic instability and shock.5 Multidetector computed tomography (CT) is the mainstay diagnosis technique with a specificity of almost 100% for diagnosis of WS, but only up to 60% for detecting underlying etiology. Follow-up imaging has been recommended to further assess a specific etiology, as small solid lesions may be obscured initially by the hematoma.1 There is no consensos regarding treatment of WS. However, the approach should be individualized to the clinical status, underlying etiology and patient’s hemodynamic stability. Endovascular treatment or surgical approach should be reserved for patient’s that fail to stabilize with conservative treatment and cases of renal neoplasms.3,6,7Mortality risk associated to WS is variable among different case series depending on underlying etiology and timely approach.3,8
We describe a case report of a 68-year-old male undergoing continuous ambulatory peritoneal dialysis (CAPD) for 5 years and taking warfarin for permanent atrial fibrillation (AF) who developed WS presenting with Lenk’s triad. The patient was managed with percutaneous drainage of the renal hemorrhage and was able to continue peritoneal dialysis (PD) in the acute phase of disease.
Unfortunately, he subsequently developed peritoneal membrane failure having to transition to hemodialysis (HD) 2.5 months after the WS episode.
The authors will review literature on SRH on end-stage kidney disease (ESKD) patients, discuss oral anticoagulation on PD patients, maintenance of PD in WS, and finally, address the consequences of WS on peritoneal membrane diffusion capacity.
CASE REPORT
A 68-year-old caucasian male undergoing CAPD was admitted on the emergency department on the 25th November 2022 due to acute abdominal pain.
The patient had a chronic kidney disease (CKD) of unknown etiology having started HD in 2008, at the age of 54, transitioning to automated peritoneal dialysis (APD) from 2008 to 2012, at which time he was submitted to deceased-donor kidney transplant. On the transplant period he developed slowly progressive graft dysfunction and graft biopsy showed chronic transplant glomerulopathy. The patient restarted CAPD in November 2017. The patient had a long-term history of hypertension, chronic systolic heart failure and permanent nonvalvular AF. His regular medication included: pantoprazol 20 mg daily, bisoprolol 2.5 mg daily, warfarin, olmesartan+amlodipine 20/5 mg daily, rosuvastatine+ezetimibe 10/10 mg daily, furosemide 40 mg 4 pills per day, folic acid and complex B vitamins daily, sevelamer 800 mg twice a day, cinacalcet 30 mg 3 times a week and paricalcitol 1 mcg 5 times a week. He had regular follow-up at the PD clinic and on a local health unit for monthly monitoring of international normalized ratio (INR), which were usually stable between 2 - 3. The patient had a history of acquired cystic kidney disease (ACKD). Last renal ultrasound on August 2022 reported atrophic native kidneys with bilateral small multiple cortical cystic lesions.
The patient was on CAPD with 4 daily exchanges of 2 L using three 2.27% glucose-based solution plus a long dwell overnight with icodextrin PD solution. His residual urine volume was 300 mL/24 hours and his average peritoneal ultrafiltration was -1800 mL/day. His weekly urea Kt/V was 2.2, residual renal function 0.58 mL/min/1.73 m2 and total creatinine clearance 63.3 mL/min/1.73 m2. On the last peritoneal equilibration test, performed in August 2022, he was a low-average transporter. The patient had no history of PD-related peritonitis.
On admission to the emergency department, the patient reported flank pain on the left side evolving for a few hours, with no aggravating or relief factor or position, and no history of trauma. The patient reported he had PD fluid on his peritoneal cavity, as per medical PD prescription, and triage sent him for nephrology evaluation.
On admission to nephrology unit, the patient stood up and had a syncope. He was pale, sudoretic, hypotensive (85/45 mmHg), tachycardic (115 bpm), febrile (auricular temperature 38.4ºC), with inadequate tissue perfusion (capillary refill test > 2 seconds). The patient recovered from the syncope in seconds after Trendelenburg position and had no cognitive compromise. He started fluid resuscitation with saline solution and peritoneal effluent was drained. Macroscopic appearance of the effluent was transparent and samples were sent for cytological and microbiologic analysis. On physical examination, the abdomen (after draining peritoneal fluid) was tender on the left quadrants and there was a palpable mass on the flank side, whose borders were ill-defined extending up to the left iliac fossa, but was tender, painful, non-mobile and not pulsatile. Entry site of peritoneal catheter showed no signs of local inflammation.
The patient reported he had been to his monthly appointment for INR monitoring 3 days earlier, presenting with INR 6.2 and was scheduled for re-evaluation of INR that day, after stopping warfarin for the last 2 days.
On initial blood work (Table 1) the most relevant findings were severe anemia (Hb 5.0 g/dL), INR 6.4 and microscopic hematuria. Last known hemoglobin value was 10.7 g/dL, a week earlier on his regular PD consultation. Emergent contrast enhanced abdominal and pelvic CT revealed large heterogeneous hematoma occupying left renal loca, with no evidence of active hemorrhage (Fig. 1).
Table 1 Blood, urinary and peritoneal fluid tests performed on admission.
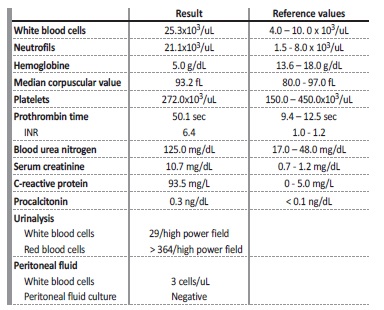
INR - international normalized ratio

Figure 1 Emergent contrast enhanced abdominal and pelvic computed tomography. On the left side sagittal view, on the right-side coronal view. Large heterogeneous hematoma occupying left renal loca with significant enhancement of peri-renal tissues and retroperitoneum, extending downwards to the iliac fossa, measuring up to 13 cm maximum diameter. There was no evidence of contrast extravasation.
The patient was assumed to have WS probably due to cystic rupture coupled with iatrogenic coagulopathy (caused by warfarin) and uremicassociated platelet dysfunction presenting with Lenk’s triad. Hypovolemic shock was managed with 5 red blood cell transfusions and coagulopathy was managed with 30 mg intravenous vitamin K and 3 units of fresh frozen plasma, total. INR was measured hourly to guide such therapy. The patient was on 6-hour manual PD exchanges with 2.27% glucose-based solutions to maintain a stable uremic milieu and guarantee adequate fluid removal during transfusional therapy. After INR levels were reversed to 2.3 and patient was hemodynamically stable (blood pressure 123/67 mmHg, heart rate 65 bpm and good capillary perfusion), he was transferred to urology care.
The patient was submitted to image-guided percutaneous drainage of renal hematoma under local anesthesia, with exit of 600 mL of liquefied blood. A pigtail drain was left in place and indication was given to clamp if there was evidence of fresh blood or hemodynamic instability that could suggest active bleeding. He was empirically started on ceftriaxone.
On the following days, the patient remained hemodynamically stable, afebrile and abdominal pain progressively improved. Hemoglobin stabilized and gradually improved with erythropoietin-stimulating agents.
Repeat multidetector CT 48 hours after percutaneous drainage showed reduction of renal sub-capsular hematoma on posterior inferior renal pole, with an extension of 10 cm maximum diameter and no signs of active bleeding. Follow-up CT excluded underlying neoplasms, arteriovenous malformations or infections, and it showed multiple bilateral multiple cortical cysts. The source of bleeding was possibly a ruptured cyst on the cortical posterior renal parenchyma. Considering the favorable clinical evolution, underlying etiology and higher surgical risks of a dialysis patient, a conservative approach was favored. The pigtail drain was removed on the 4th day after percutaneous drainage when exudate production was < 50 mL/24 hours. Repeat CT showed gradual reduction of renal hematoma total dimensions with no signs of active bleeding.
Throughout his hospitalization the patient maintained CAPD with 4 exchanges per day, three 2.27% glucose-based solutions and overnight dwell with icodextrin solution, with daily ultrafiltration exceeding 1L, stable body weight, good blood pressure and adequate control of extracellular volume.
The patient was discharged on his 10th day of hospitalization and oral anticoagulation was not re-introduced. Two months later, repeat CT showed renal subcapsular and another retroperitoneal hematomas (Fig. 2).

Figure 2 CT showing renal subcapsular hematoma measuring 2.7 cm and another retroperitoneal hematoma on posterior para-renal location measuring 5.5 cm maximum diameter. There were no signs of active extravasation and peritoneum was drained immediately before for better visualization.
On follow-up PD consultations, the patient had become anuric, developing uremic symptoms and his peritoneal ultrafiltration was gradually decreasing, coupled with increasing blood urea nitrogen levels, hyperphosphatemia and higher erythropoietin dose requirement for anemia control. His weekly urea Kt/V 2 months after WS had decreased to 1.6, not only due to the fact that he became anuric, but also due to reduced peritoneal clearance. The patient transitioned to HD 2.5 months after renal hemorrhage episode due to dialysis inadequacy. Warfarin was indefinitely stopped.
DISCUSSION
Spontaneous atraumatic renal hemorrhage of unknown cause was first described by Carl Reinhold August Wunderlich in 1856 as a spontaneous renal capsule apoplexy.9 It appears in literature as WS or SRH. We have previously described the major systematic reviews on SRH and how they are biased as they excluded patients on dialysis or anticoagulation.3 Mao Y et al in 2017 published a single-center retrospective analysis of SRH cases10 which included patients for their imaging criteria on CT (n=78). In this case series, coagulation abnormalities were identified in 27% of cases and ruptured renal cysts as the underlying cause of WS in 13% of cases. They concluded that renal hemorrhages related to coagulation abnormalities were more likely to cause more diffuse perinephric hematomas affecting the pararenal space. Trischott et al in a case report description, referred to a PubMed search of all WS described in association with anticoagulation up to 2015. In their analysis, anticoagulation caused 31 cases of WS, mostly with heparin (45%), warfarin (29%) and antiplatelet therapy (26%). Risk factors for such bleeding complication in their analysis were older age, CKD and concomitant treatment with non-steroidal inflammatory drugs.11
Malek-Marin et al described a case series of patients undergoing dialysis and reviewed the literature for SRH on dialysis patients. He concluded that SRH occurs much more frequently in HD as compared to PD and ACKD was the common underlying etiology in such population.
They noticed that duration of renal failure seems to be a risk factor for ACKD leading to renal hemorrhage and hypothesized that the more cortical location of the cysts in ACKD predisposed more to hemorrhage when compared to autosomal dominant polycystic kidney disease (ADPKD). Most episodes of bleeding occurred during or shortly after dialysis session, which may reflect the role of heparin.12
A search on PubMed of SRH in PD patients after the year 2000 reported 3 single-case reports. The first, reported in 2006 by Borrás M et al, a patient on CAPD and had WS secondary to ACKD with a transitory period of hematic effluent, which they interpreted in the context of proximity of the cyst and peritoneal membrane. This patient was managed with nephrectomy and was able to maintain PD. The second case, reported by Biyik Z et al in 2015, the patient presented with hematic peritoneal drainage but no signs of hemodynamic instability.
CT revealed a suspicious exophytic cystic lesion and the patient underwent radical nephrectomy with resolution of retroperitoneal hemorrhage. The lesion was later attributed to ACKD.14 The third case, reported in 2021 by Wen TC et al, reflected a young patient that had transition a few months before to HD due to encapsulating sclerosing peritonitis.15
In our case report, the patient was able to be hemodynamically stabilized and coagulation abnormalities were rapidly corrected, however the hemorrhage dimensions were very large and despite there was no suspected active extravasation on CT, the surgical team opted for percutaneous drainage. This approach took into consideration that the patient was on PD and there could be a conflict of space, and the fact that with such hematoma dimensions and coagulation abnormality present on admission, there was an uncertainty regarding the presence of active bleeding.
Another issue in our case report is that patient was on warfarin for atrial fibrillation for more than 2 years and despite regular monitoring of his INR with stable levels, he had sudden severe supra-therapeutic levels leading to a stern bleeding complication. Vitamin K antagonists are known to have many variations related to changes in medications, diet, alcohol use and other comorbidities. In our case report, we did not identify a specific cause that can justify such sudden alteration in INR control.
Despite growing evidence that newer oral anticoagulants may be safer and equally effective in prevention of thrombotic events in ESKD, warfarin remains the anticoagulant most commonly used. When anticoagulants are used for prevention of cardioembolic ischemic events attributable to nonvalvular AF, we should remember that such risk in dialysis population remains unclear, and therefore, initiation of anticoagulation should probably obey the primum non nocere principle, meaning, we consider the patient has low risk of bleeding.16 Of course this adds to a bigger issue, which is, what is the best bleeding risk score in ESKD. A promising risk score is the Valkyrie trial Dialysis Risk Score, which was used in HD patients only.17
Drug pharmacokinetics and pharmacodynamics differs between HD and PD. Few studies evaluated anticoagulation on such population, however, PD patients in general seem to have less bleeding complications than HD patients, possibly due to less exposure to heparin, more stable hemodynamics and electrolyte levels.18 Phan et al published
a study in 2019 regarding anticoagulation with warfarin for AF on PD patients. They concluded there was no association between warfarin treatment and mortality risk as compared to PD patients not taking warfarin. This was a large-cohort observational retrospective study therefore advice is cautioned for taking conclusions, as possible confounding factors may not have been taken into account. Interestingly, warfarin use in this study was not associated with reduction of risk for ischemic stroke.19
On previously reported cases of WS on PD patients, hemoperitoneum was found, despite the fact that, in theory, retroperitoneum does not contact with peritoneal cavity. Our patient had clean peritoneal effluent throughout course of disease, however he became anuric, probably due to ischemic renal injury in the context of shock. Kt/V reduction, was not exclusively due to anuria, as creatinine peritoneal clearance had also drastically reduced. The authors hypothesize that inflammation and fibrosis, on the course of hematoma reabsorption, extended to visceral peritoneum due to its anatomical proximity, reducing the available peritoneal surface area and its ultrafiltration properties, leading to peritoneal membrane failure. Nevertheless, the patient had a total of 9 years on PD, which is considerable.13
KEY MESSAGES:
1) WS or SRH occurs infrequently but can have serious complications, especially in CKD and ESKD patients due to their complexity.
2) ACKD and coagulation abnormalities (including iatrogenic) may be more common underlying causes of SRH in ESKD population than previously reported.
3) Acquired kidney cystic disease has a risk of rupture (higher than ADPKD cysts) which is important to surveil in dialysis patients, especially after a long-term renal replacement therapy.
4) Anticoagulation for prevention of cardioembolic complications of nonvalvular AF in ESKD patients should be questioned as there is no clear evidence of benefit.
5) PD patients, despite apparently lower bleeding risk when compared to HD patients, are still high-risk patients for their uremic-milieu and warrant for close monitoring and vigilance when using of anticoagulants.















