INTRODUCTION
Antibody-mediated acute rejection (ABMR) occurs in 5%-7% of renal transplant patients; its incidence increases to 40% in highly sensitized recipients.1 Even with optimum treatment, the risk of graft failure increases both on the short and on the long term.1 Risk factos for ABMR include blood group incompatibility, human leucocyte antigen (HLA) mismatching, presence of human donor-specific antibodies (DSA), long ischemia time and delayed graft function.2 Current evidence indicates that a substantial proportion of acute and chronic renal allograft rejection is due to specific antibodies to donor antigens.2 Significant progress has been made in the diagnosis of ABMR, mainly driven by the development of sensitive assays for the detection of DSAs against human leukocyte antigens (HLA).3,4
Some patients meet the histological criteria for ABMR but do not have detectable DSA. This could be explained by the presence of injuriou antibodies that remain undetected due to the limitation of the current testing methods.5,6 We report a case of a kidney transplant recipiente with a delayed graft function due to an antibody-mediated rejection without detectable HLA-DSAs.
CASE REPORT
A 69-year-old Caucasian man with end-stage renal disease caused by diabetic nephropathy received a first cadaveric kidney transplant in March 2022. It was a donor with expanded criteria who had a brain death. This patient had no residual renal function and was on hemodialysis for 5 years. His virology status for hepatitis C and B, as well as human immunodeficiency virus was negative. There was no history of recent vaccinations or major infections and autoimmune diseases. HLA typing exhibited four mismatches (one in HLA-A, 2 in HLA-B and one in HLA-DR). The virtual panel-reactive antibody was 29.17%, without history of blood transfusions. Pre-transplant complement-dependent cytotoxic and flow cytometry cross-matches were negative for B and T lymphocytes. Induction immunosuppressive therapy consisted of basiliximab, methylprednisolone, mycophenolate mofetil and tacrolimus.
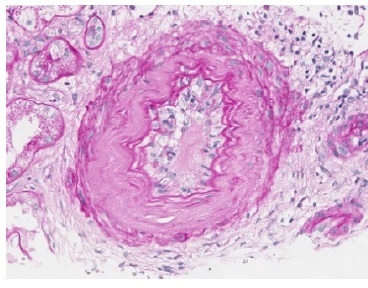
The transplant surgery was unremarkable with a cold ischemic time of 10 hours and a warm ischemic time of 10 minutes. The patient presented immediate urinary output but delayed graft function and dialysis dependency since day one post-transplant. The tacrolimus trough was at a supratherapeutic level, and a progressive adjustment was made for a desired level of approximately of, approximately, 10 ng/mL. On the 8th post-operative day, an allograft biopsy was performed and revealed severe peritubular capillaritis, severe glomerulitis, moderate arteritis, acute tubular necrosis in approximately 50% of cortical tubules, focal signs of small vessel acute thrombotic microangiopathy and a positive C4d by immunohistochemistry (Figs 1,2 and 3). In the same day, post-transplant T- and B-cell cytotoxic and flowcytometry cross-matches were found to be again negative and HLADSAs were also negative. The patient fulfilled the histological criteria for an active ABMR without detectable HLA-DSAs. In the following day, the patient began treatment, initially favouring a more conservative therapeutic approach, taking into account the patient’s advanced age and highly fragile state. He was initially treated with intravenous methylprednisolone (500 mg a day, three administrations) and thymoglobulin (cumulative dosage of 450 mg - only six administrations due to the onset of thrombocytopenia), remaining dialysis-dependent and without urinary output improvement. On 18th day post- transplant, a second allograft biopsy revealed only mild peritubular capillaritis and glomerulitis with a positive C4d on immunohistochemistry. We performed 6 plasma exchange treatments in alternate days (using 1.2 plasma volumes and mainly 5% human albumin, but also human plasma as replacement fluids) and immunoglobulin (total of 2 g/kg).
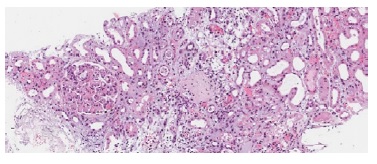
Figure 1 H&E 100x. Two glomeruli are shown, one with global glomerulosclerosis and the other with severe glomerulitis. Severe acute tubular necrosis is also present with necrotic tubular epithelial cells casts observed in distal tubuli.
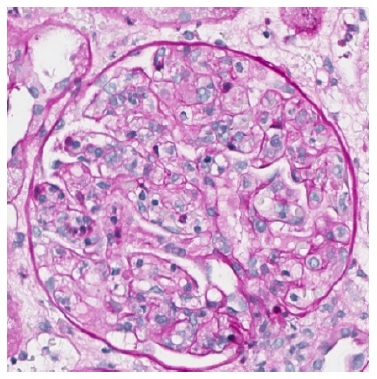
Figure 3 PAS 400x. Severe glomerulitis with several capillary loops completely occluded by leukocyte infiltration and endothelial cell enlargement.
The patient gradually improved his urinary output and became dialysis independent on the 33th post-transplant day. The patient was discharged on the 50th post-transplant day with a serum creatinine of 6.37 mg/dL. In the following weeks, the level of serum creatinine slowly decreased and, six months post-transplant, remains stable at 2.0 mg/dL. No infections or other side effects of treatment were observed and he maintains immunosuppression with mycophenolate mofetil (750 mg, twice daily), tacrolimus (level of approximately of 8 to 10 ng/mL) and low dose prednisolone (5 mg/day).
DISCUSSION
ABMR is the most important cause of kidney allograft failure and dysfunction after kidney transplantation.7 HLA-DSAs are considered to have major importance in the pathogenesis of ABMR but often remain undetectable in the serum of patients with proven ABMR. In cases of proven ABMR, HLA-DSA status may affect prognosis and influence therapeutic choices.2,8
There are possible causes of failure to detect pathogenic HLA-DAS like failure to type all 11 HLA loci (A, B, Cw, DRβ1, DR51, DR52, DR53, DPα, DPβ, DQα, and DQβ) in donor and/or recipient, high cut-off for positivity and mediation by donor specific memory B cells.9 The phenomenon of HLA-DSA directed against a very public epitope expressed on many SAB diluting down the MFIs of any 1 bead was another cause to have in mind but it was recently disaffirmed in a study of Guillaume C et al.10 In our particularly case, all 11 loci were typed both in donor and recipient and high cut-off for positivity was not a problem reported by our histocompatibility center. The mediation by donor specific memory B cells represents a major challenge in kidney transplantation. Historically, due to the lack of appropriate and routinely applicable assays to determine the presence and HLA specificity of alloreactive memory B cells, their contribution to the humoral alloimmune response has clinically often been suspected but could not be determined. Alloreactive memory B cells can nowadays be detected by using several techniques, such as T/B ELISpot assays, but in challenging cases additional molecular analysis at histopathology level (for example, IFNy-inducible, natural killer cell and T-cell transcripts) could be useful.11,12
The role of non-HLA antibodies has already been evaluated and described in literature, such as AT1R-Ab, ETAR-Ab, MICA-Ab or anti-EC antibodies detected with crossmatches, and found they could not explain ABMR with negative HLA-DSAs.13 Their presence was investigated in this case and is negative.
The pathophysiology of ABMR without HLA-DSAs remains nuclear and there is little literature on the phenotype and outcome of this entity. It is demonstrated that HLA-DSA negative ABMR is observed relatively frequently after transplantation, and that the number of these cases even outnumbered the number of cases with detectable DSAs.2,14
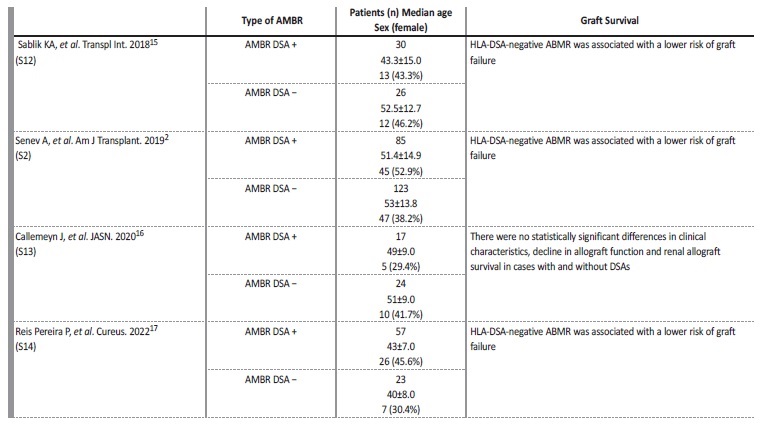
We report a case of an ABMR without detectable DSAs that happened in an older male patient, in the period immediately after kidney transplantation, who was a first-time recipient. According to the literature, patient phenotype with ABMR without HLA-DSAs appears different from HLA-DSA positive ABMR, with patients being frequently first kidney transplant recipients, older, and with more HLA mismatches, particularly in locus A.2 The risk of graft failure is also significantly lower in patients without HLA-DSAs in comparison to patients with similar histology but with HLA-DSAs. Some of these cases could be related to concomitant T-cell mediated rejection, but the subgroup of patients with ABMR in the absence of HLA-DSAs and T-cell mediated rejection remains poorly understood. A few cases are cases are now described in literature (Table 1).2,15-17
Patients with ABMR in the absence of HLA-DSAs have a diferente evolution after their first graft biopsy. These patients have significantly fewer subsequent biopsies with ABMR than HLA-DSAs positive patients, and less development of chronicity.2,15-17
In the absence of DSAs, C4d deposition in PTCs is significantly less and not associated with worse graft outcome.2 Although the association between C4d deposition in PTCs and HLA-DSA positivity is confirmed, using C4d deposition in PTCs as proxy for circulating HLA-DSA, as suggested in the recent update of the Banff classification, did not contribute to the prognosis of graft function and failure, independente of the threshold used.2,18These finding questions the last Banff 2017 update of the diagnostic criteria for ABMR. In summary, patients with ABMR but without HLA-DSAs represent a distinct, often transiente phenotype with superior graft survival compared to patients with circulating HLA-DSA and fully developed ABMR by Banff criteria.2
As previously described, our patient was treated with plasma exchange sessions and immunoglobulin with a surprisingly good clinical and histological response to treatment. Currently, the ideal treatment remains unknown, with little evidence to supporting the use of a specific therapy and we rely on the use of apheresis for alloantibody depletion and intravenous immunoglobulin (referred to as standard of care), preferentially in early active ABMR. However, there are now several promising treatment approaches in the pipeline, which are being trialed in phase II and III studies. These include interleukin-6 antagonism, CD38- targeting antibodies, and selective inhibitors of complement. On the basis of the information that has emerged so far, it seems that innovative treatment strategies for clinical use in ABMR, with and without detectable HLA-DSAs, may be available within the next 5-10 years.19
Actually, there are studies that aim to compare the effect of various ABMR treatment approaches on allograft survival and to compare treatment effects in the presence or absence of HLA-DSAs. The results of this studies suggest that long-term application of intravenous immunoglobulin is more favorable for HLA-DSAs positive recipients, whereas intensification of maintenance immunosuppression is more effective for recipients with HLA-DSAs negative ABMR.20
By bringing this clinical case to light, our aim is to highlight this new entity that is increasing, with an urgent need for a better characterization of the different forms of ABMR based on pathophysiology, histology, as well as clinical and genetic phenotypes. Further work is needed in order to elucidate the pathophysiology of ABMR in the absence of HLA-DSAs and novel markers are needed for an early diagnosis.