INTRODUCTION
Around 700 million people worldwide have CKD, which is an importante cause of morbidity and mortality, partly explained by the prevalence of cardiovascular (CV) disease in this population.1,2 The 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines define CKD as reduced glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 and/or albuminuria (≥30 mg/d), persistent for at least 3 months, regardless of the underlying cause.3,4 Clique ou toque aqui para introduzir texto. Currently, renin-angiotensin system (RAS) blockers are the gold-standard in treatment of CKD progression, by reducing systemic and intraglomerular pressures.5
SGLT2i, firstly introduced as therapy for type 2 diabetes (T2D), act by blocking glucose and sodium reabsorption in the renal proximal tubule cells through the SGLT2 transporter, leading to enhanced urinary glucose and sodium excretion. Unexpectedly, recent trials showed that SGLT2i have positive CV and metabolic effects independently of their glucose-lowering outcomes. Accumulating evidence suggests that CV and renal protection associated with SGLT2i is unrelated to the hypoglycaemic effect. Indeed, these have been proven to reduce body weight (BW), systemic blood pressure (BP) and systemic inflammation which impact in these outcomes.6-12
There are several proposed mechanisms underlying renal protection, such as control of glomerular hyperfiltration by decreased sodium reabsorption and subsequent glomerular afferent arteriolar vasoconstriction,13-15 and reduction in intraglomerular pressure providing na antiproteinuric effect.16,17
This systematic review aims to analyse the effect of SGLT2i on renal outcomes in patients without DM.
METHODS
This systematic review was performed in a PRISMA compliant manner. PubMed, Medline and CENTRAL (Cochrane Controlled Register of Trials) databases were searched on 30 November 2022. Search was conducted using the terms “Sodium-Glucose Transporter 2 Inhibitors” OR “canagliflozin” OR “dapagliflozin” OR “empagliflozin” AND ‘“without diabetes” OR “non diabetic”.
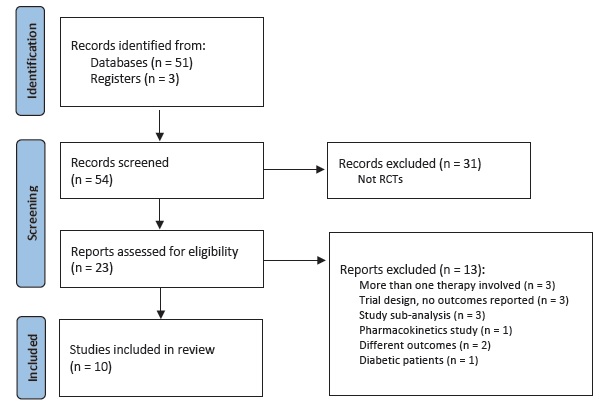
Fifty results were retrieved from January 2013 to December 2022. We included randomized trials only. Clinical trials were selected to identify if subgroup analysis of patients without diabetes was performed. Inclusion criteria were randomized trials evaluating renal outcomes in patients without DM. Exclusion criteria were studies on diabetic population only, animal or in vitro studies, and protocols or comments on the trials. Of the twenty-two randomized trials found, we included ten.
Renal outcomes included changes in eGFR, albuminuria or proteinuria and progression to end-stage renal disease (ESRD) as detailed in each study. Death from renal or CV causes was also assessed.
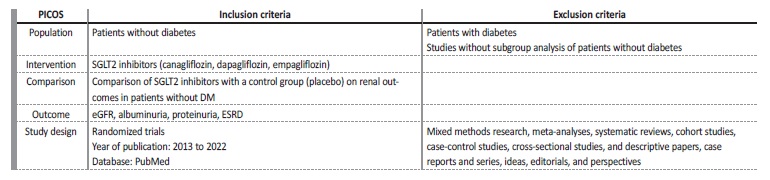
Four reviewers independently executed the literature search and data extraction. Data relating to blinding and withdrawals were extracted to assess risk of bias. We collected data on the drug name, dosage and frequency, control group, and length of intervention. This systematic review was not registered. Population, intervention, comparison, outcome, study design, inclusion and exclusion criteria are detailed in Table 1.
RESULTS
Study selection
The PRISMA flowchart is presented in Fig. 1. The ten trials comprised a combined cohort of 26 298 patients. Among the ten studies, four included patients with HF, one included patients with obesity and five studies included patients with CKD. Among the SGLT2i used in the trials, five studies used dapagliflozin, four used empagliflozin and one used canagliflozin. Dapagliflozin and empagliflozin were administered at a dosage of 10 mg, while canagliflozin was administered at dosages of 50 mg, 100 mg and 300 mg in the same trial. All regimens were given once daily and placebo controlled.
Study characteristics
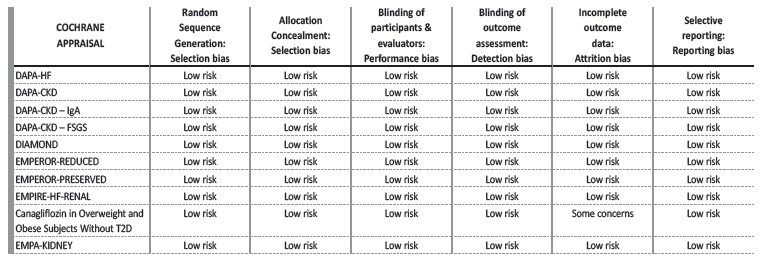
We collected data of 13 399 patients without DM. Mean age was 59.6±8.3 years, mean GFR was 53.7±11.4 mL/min/1.73 m2 and mean follow-up was 17.4±10.6 months. The Cochrane risk of bias tool was used to critically evaluate the selected articles.18
The bias risk assessment looked at six causes of potential bias and a summary for each trial is figured in Table 2. Inclusion and exclusion criteria of the selected studies are detailed in Table 3. Table 4 provides a summary of the studies’ characteristics and outcomes.
Table 3 Inclusion and exclusion criteria
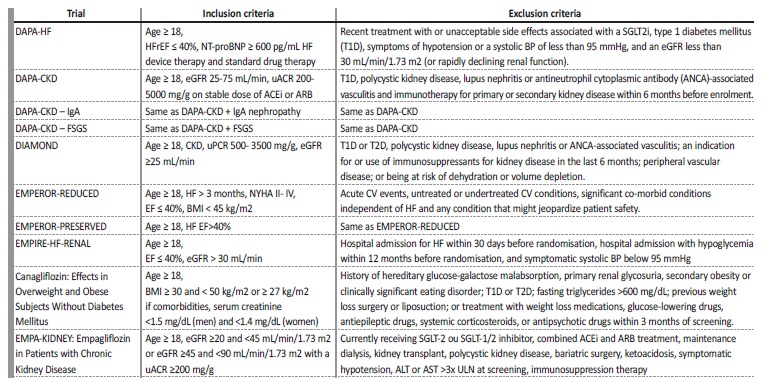
ACEi - angiotensin-converting enzyme-inhibitor, ALT - alanine transaminase, ARB - angiotensin receptor blocker, AST - aspartate transaminase, BW - body weight, CV - cardiovascular, eGFR - estimated glomerular filtration rate, FSGS - focal segmental glomerulosclerosis, HFrEF - heart failure with reduced ejection fraction RAS - renin-angiotensin system, SGLT - sodium-glucose cotransporter, uACR - urinary albumin-to-creatinine ratio, ULN - upper limit of normal , uPCR - urinary protein excretion.
Table 4 Summary of the included studies
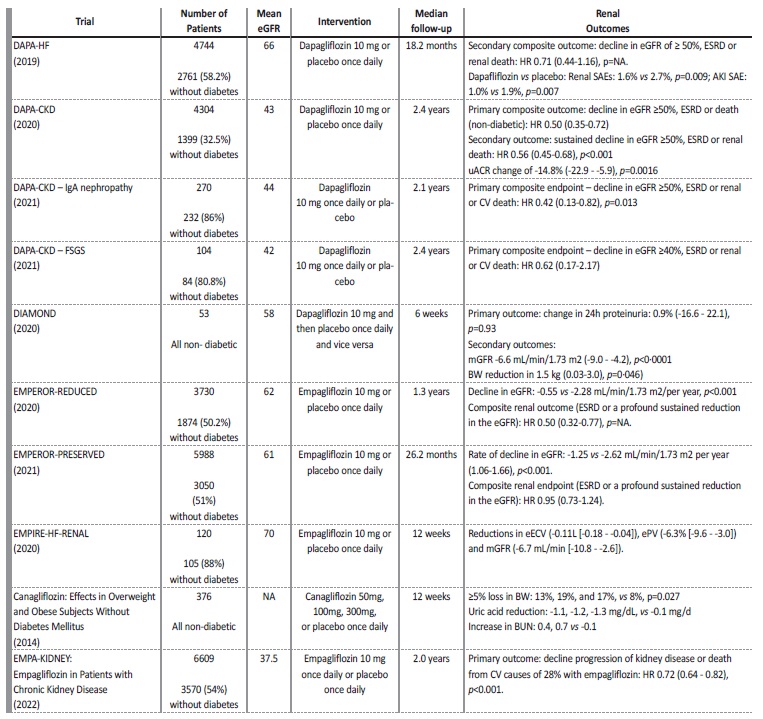
AKI - acute kidney injury, BW - body weight, BUN - blood urea nitrogen, CV - cardiovascular, eECV - estimated extracellular volume, eGFR - estimated glomerular filtration rate, ePV - estimated plasma volume, ESRD - End-stage renal disease, HR - heart rate, NA - not applicable, SAE - serious adverse event, uACR - urinary albumin-to-creatinine ratio.
All studies had a low risk of bias. One study (“Canagliflozin in Overweight and Obese Subjects Without Diabetes Mellitus”) contributed to a potential attrition bias due to high dropout rates, as 25% of the participants did not complete the study.19
Outcomes
In the DAPA-HF trial there was less worsening renal function (defined as a sustained decline in the eGFR of ≥50%), ESRD or death from renal causes on the dapagliflozin group though this difference was not significant (0.8 vs 1.2 events/100 patient-year, HR 0.71 (0.44 to 1.16), p value not applicable).10
The DAPA-CKD study demonstrated that dapagliflozin reduced the risk of decline in eGFR of at least 50%, or ESRD or death from renal or CV causes (HR 0.61 (0.51 to 0.72) p<0.001), which was lower in the non-diabetic group (HR 0.50 (95% CI, 0.35 to 0.72)).
Reduction in eGFR was noticeable in the first two weeks of treatment (-3.97±0.15 vs -0.82±0.15 mL/min/1.73 m2), then, the anual decline in eGFR was lower with dapagliflozin (-1.67±0.11 vs -3.59±0.11 mL/min/1.73 m2).16
An analysis from the DAPA-CKD trial revealed that urine albumincreatinine ratio (uACR) was reduced with dapagliflozin by 29.3% (95% CI, -33.1 to -25.2, p<0.0001).20 Reduction was higher in patients with T2D (-35.1% (95% CI, -39.4 to -30.6, p<0.0001) vs -14.8% (95% CI, -22.9 to -5.9, p=0.0016)). Additionally, greater reductions in uACR were significantly associated with smaller eGFR decline throughout follow-up (-3.06, 95% CI, -5.20 to -0.90; p=0.0056).16
In the DAPA-CKD-IgA trial, dapagliflozin reduced the risk of sustained decline in eGFR of at least 50%, ESRD, or death from renal or CV causes in non-diabetic patients (HR 0.42 (95% CI, 0.13 to 0.82, p=0.013)). In the first two weeks of treatment there was an eGFR reduction in the dapagliflozin group (-3.4 [±0.4] vs -0.5 [0.4] mL/ min/1.73 m2), then, annual eGFR change was lower with dapagliflozin (-2.2 [0.5] and -4.6 [0.47], respectively).21
In the DAPA-CKD-FSGS trial, dapagliflozin reduced the risk of sustained ≥40% decline in eGFR, or ESRD or death from renal or CV causes (8.9 vs 11.9%; HR 0.62, [95%CI 0.17-2.17]). Also, dapagliflozin led to an acute decline in eGFR (-4.5, 95% CI, -5.9 to -3.1 vs -0.9, 95% CI, -2.1 to 0.4 mL/min/1.73 m2/two weeks), and lower annual rates of eGFR decline (-1.9 (95% CI -3.0 to -0.9) vs -4.0 (95% CI -4.9 to -3.0) mL/min/1.73 m2/year). Although not statistically significant, the total slope of decline was lower with dapagliflozin (-3.7 (95% CI, -4.8 to -2.6) vs −4.2 (95% CI, -5.2 to -3.3) mL/min/1.73m2/year).22
In the DIAMOND trial, dapagliflozin was associated with a percentage change in 24 hours proteinuria of 0.9% (-16.6 to 22.1, p=0.93). Also, there was a reduction of eGFR by -6.6 mL/min per 1.73 m2 (-9.0 to -4.2; p<0.0001) at week 6 with dapagliflozin which was reversible six weeks after discontinuation.7
In the EMPEROR Reduced trial, empagliflozin was associated with a slower rate of decline in the eGFR, regardless of the presence or absence of diabetes (-0.55 vs -2.28 mL/min/1.73 m² per year, p<0.001). Composite renal outcome (ESRD or sustained reduction in the eGFR) was also less frequent with empagliflozin (HR 0.50; 95% CI, 0.32 to 0.77, p value not shown). Difference between diabetic and non-diabetic patients was not specified.9
In the EMPEROR Preserved trial, empagliflozin had a slower rate of decline in eGFR (-1.25 vs -2.62 mL/min/1.73 m² per year (95% CI, 1.06 to 1.66; p<0.001), and was associated with a lesser risk of a composite renal outcome (ESRD or a profound sustained reduction in the eGFR), although not-significant (HR 0.95 (0.73 to 1.24)), which was similar in patients with or without diabetes.23
In the EMPIRE-HF-RENAL trial, empagliflozin was associated with a decline in estimated extracellular volume (eECV) (mean difference -0.11L [-0.18 to -0.04]) and eGFR (mean difference -6.7 mL/min [-10.8 to -2.6]) at 12 weeks.24
In the canagliflozin in overweight and obese subjects without diabetes trial, treatment with canagliflozin resulted in an increase in urinary glucose excretion (UGE)/creatinine ratio in a dose-dependent manner. Canagliflozin also produced a ≥ 5% loss in BW (p=0.027), and reductions in systolic BP in a dose-dependent manner. Also, canagliflozin was associated with modest decreases in eGFR, and increases in blood urea nitrogen (BUN) at a short follow-up.19
The EMPA-KIDNEY trial demonstrated a reduction in the risk of progression of kidney disease or death from CV causes of 28% with empagliflozin (p<0.001). When these components were analysed separately, empagliflozin remained associated with a significant reduction in the rate of kidney disease progression with relative risk reduction of 29% (HR 0.71; 95% CI, 0.62 to 0.81). There was a consistente benefit of empagliflozin treatment among patients with or without diabetes and regardless of eGFR. Additionally, it seems to suggest a greater risk reduction benefit among patients with higher uACR (> 300 mg/g). In the empagliflozin group there was an acute initial decline in eGFR in the first 2 months. However, the annual rate of eGFR decline was slower with empagliflozin by 0.75 mL/min/1.73 m2 (95% CI, 0.54 to 0.96), including in the subgroup of patients with a low uACR.25
Adverse effects
Concerning adverse effects, in the DAPA-HF trial, dapagliflozin was associated with less renal serious adverse events (SAEs) than placebo 1.6 vs 2.7% (p=0.009). Acute kidney injury (AKI) was the most common renal SAE.10
The incidences of adverse events (AEs) and SAEs were similar overall in the dapagliflozin and placebo groups in the DAPA-CKD trial and subgroup analysis. Neither diabetic ketoacidosis nor severe hypoglycaemia was observed in participants without diabetes.16,21,22
In the DIAMOND trial, dapagliflozin resulted in more AEs than placebo, 32% vs 25%, respectively. Concerning patients treated with dapagliflozin, one developed AKI, one had urinary tract infection, and another a genital infection. There were two SAEs, one with placebo and the other with dapagliflozin.7 SAEs occurred in 41.4% of patients treated with empagliflozin vs 48.1% with placebo in the EMPEROR Reduced trial.9 In the EMPEROR Preserved trial, SAEs occurred in 47.9% patients with empagliflozin and in 51.6% with placebo.23
In the EMPA-KIDNEY trial more patients had lower limb amputations and ketoacidosis, however, this just occurred in 0.8% and 0.6% of patients, respectively. Uncomplicated genital and urinary tract infections (UTI) and hypotension were more common in patients treated with empagliflozin.9,23,25
In the EMPIRE-HF-RENAL, empagliflozin was associated with SAEs in 8% of patients versus 5% with placebo. The most common AE was UTI (7% vs 5%), with one patient in the empagliflozin group requiring hospital admission.24
The overall incidence of AEs was comparable in all treatment groups with canagliflozin.19
DISCUSSION
In this systematic review of randomized trials of patients without DM treated with SGLT2i (dapagliflozin or empagliflozin), there is a tendency for lesser risk of sustained eGFR reduction, progression to ESRD or renal death.
Initially studied in the diabetic population, SGLT2i showed that beside the CV benefit, they could reduce progression to macroalbuminuria, doubling of serum creatinine, ESRD or death from kidney causes by 40% in the EMPA-REG trial.26Subsequently, the CREDENCE trial also proved the renoprotective benefits of SGLT2i in a CKD diabetic patient cohort.27 The trial was terminated early because of significant efficacy of the drug. The relative risk (RR) of the renal composite outcome of ESRD, doubling of the creatinine level and renal death was 34% less (HR 0.66, p<0.001), and the RR of ESRD was lower by 32% (HR 0.68, p=0.002). CREDENCE, EMPA-REG also reported an early decline in eGFR (3 to 6 mL/min/1.73 m2) compared to placebo.26,27 This early reduction was typically observed between the second and fourths weeks of therapy, with partial recovery by week 12, and followed by na attenuation of the slope of eGFR decline compared with placebo after 52 weeks. As what happens with RAS inhibitors, eGFR decline and longterm clinical benefits seem to coexist with SGLT2i.28,29
In our analysis, in CKD and proteinuric patients with eGFR ≥ 25 mL/ min/1.73 m2 treated with dapagliflozin there was a statistically significant lesser risk of sustained eGFR reduction, progression to ESRD or death from renal causes in the long-term - DAPA-CKD, which was also evidente in subgroup analysis - DAPA-CKD-IgA, DAPA-CKD-FSGS.16,21,22 Empagliflozin also seems to lead to a statistically significant reduction in the rate of decline in eGFR per year - EMPEROR PRESERVED, EMPEROR REDUCED, EMPA-KIDNEY.9,23,25 Indeed, the EMPA-KIDNEY trial was stopped early due to clear positive efficacy of empagliflozin in CKD patients. This trial included patients with eGFR ≥ 20 mL/min/1.73 m2 and wide ranges of albuminuria and demonstrated a significant risk reduction of kidney disease progression or CV death.32 However, the benefit appears to be higher in patients with uACR > 300 mg/g.
SGLT2i increase the sodium and chloride reaching the macula densa, by inhibiting sodium and glucose reabsorption in the proximal tubule. This results in vasoconstriction of the afferent arteriole and a reduction in the intraglomerular pressure and GFR (as seen with RAS inhibitors), and reduction in albuminuria.8 Natriuresis and intravascular volume reduction also decrease BP and probably contribute to the initial decline in GFR.30 Indeed, despite the initial decline in GFR, the hemodynamic effects of SGLT2i result in reduction in intraglomerular pressure which may promote long-term renoprotection and lesser risk of CKD progression.30,31 This initial GFR decline but with longterm benefit was demonstrated in the EMPEROR Reduced, EMPAKIDNEY and DAPA CKD trials.16,23,25
Concerning the antiproteinuric effects, the DIAMOND trial was unsuccessful in achieving a significant decrease in urine protein excretion, but this might be explained by the short duration of treatment - six weeks.7 In contrast, a sub-analysis from the DAPA-CKD trial reported a significant decline of urine protein excretion with dapagliflozin administered for a mean period of 2.4 years. In patients with CKD, with and without T2D, dapagliflozin was associated with a significant reduction in albuminuria, which was greater in patients with T2D. Greater reductions in albuminuria were associated with a reduced eGFR decline.20 This was also demonstrated in a pooled analysis of phase three randomized trials. Over 102 weeks, dapagliflozin 5 mg and 10 mg reduced uACR by 47% and 38%, respectively, compared to placebo.17 Proteinuria reduction may be explained by the reduction of intraglomerular pressure. Also, in an animal study, dapagliflozin was associated with a decrease in proteinuria, glomerular lesions, and foot process effacement. In vitro, dapagliflozin decreased albumin-induced cytoskeletal rearrangements in cultured human podocytes.33 Weight loss has been consistently observed with SGLT2i therapy.
The reduction of BW is about 1.5-2 kg and is dose-dependent.34-38. This is resultant from glucose excretion in the kidney (about 60-100 g of glucose per day), resulting in calorie loss and osmotic diuresis.39 Most important, the weight loss is mainly due to loss in subcutaneous and visceral adipose tissue, rather than lean tissue.40 In obese individuals without diabetes, co-administration of SGLT2i with a GLP1 receptor antagonist can reduce BW by 4.5 kg, and this can be maintained for up to 1 year.39 In this systematic review, we report that SGLT2i were associated with weight loss in patients without DM, treated with dapagliflozin, empagliflozin and canagliflozin.7,10,19,23,24
As described in previous studies, uncomplicated genital infections, UTIs and hypotension were more common in patients treated with SGLT2i. Nevertheless, the incidence of AEs was low overall and similar in both groups.
Should SGLT2i be given to all CKD patients?
SGLT2 inhibitors, which were originally developed to treat T2D, have demonstrated benefits in patients with albuminuria with or without diabetes including patients with IgA nephropathy, FSGS, and HF. The use of dapagliflozin and empagliflozin can be extended to eGFRs down to 25 and 20 mL/min/1.73 m2, respectively, although starting at higher eGFRs may result in more beneficial effects.
Regarding albuminuria, the benefit appears to be higher with significant risk reduction of kidney disease progression or CV death in patients with uACR > 300 mg/g. In patients with CKD, non-diabetic and without albuminuria, the slope of eGFR was slower. Considering side effects, SGLT2 inhibitors appear to be well tolerated with a low incidence of adverse effects. Also, clinicians should be alert to the initial eGFR decline associated with starting SGLT2 inhibitors to avoid discontinuation. These drugs are modifying the management of CKD patients, and further studies are ongoing to define their role in patients with GFR < 20 mL/min/1.73m2, and in dialysis and kidney transplant patients. The results are encouraging, and the next few years will bring clarity on the effects of SGLT2 in all CKD patients.
This systematic review has several limitations to be noted. Firstly, we used published summary data rather than individual patient data which prevented a statistical analysis. Secondly, the small populations in some studies may have limited their results. The heterogeneity in evaluated outcomes limits the accurate comparison and generalization of the results. Finally, SGLT2i effect on each renal outcome is still missing since most studies focused on a composite renal outcome.
CONCLUSION
The impact of SGLT2i in CV outcomes is widely described in patients with and without diabetes. In CKD patients, the beneficial effect in reducing eGFR decline and CV mortality has also been demonstrated but appears to be greater in patients with diabetes or patients with higher degrees of albuminuria.
Further studies, specifically with long-term follow-up, are required to accurately assess the impact of SGLT2i in non-diabetic and nonproteinuric CKD patients, and in end stage kidney disease patients.