INTRODUCTION
Rituximab is an anti-CD20 monoclonal antibody that specifically targets CD-20 B-lymphocytes at an intermediate level of their development, resulting in rapid B-cell depletion. It does not act on neither precursors nor mature B cells since these do not show the CD20 marker.1 The suppression of these cells usually lasts from 6 to 12 months, although there have been reports of this depletion lasting more than 2 years.2
This monoclonal antibody was first used in relapsing and refractory indolent non-Hodgkin lymphoma in 1997 and has since been used regularly in other B-cell malignancies. Later, during the 21st century, rituximab started showing benefits in the treatment of some autoimmune disorders, such as rheumatoid arthritis.3 Its mechanism of action includes complement mediated cytotoxicity, antibody mediated cytotoxicity and B cell apoptosis. While in antibody-mediated diseases the effect of rituximab is straightforward, in others such as minimal change disease (MCD) the mechanism is still not well understood.2,4
Glomerulonephritis can range from asymptomatic urinary abnormalities to severe acute kidney injury and risk of end-stage kidney disease (ESKD) with the need for renal replacement therapies. While many forms can be managed with supportive measures or treated with corticosteroids, some glomerular diseases will require other immunosuppressive therapies such as cyclophosphamide, calcineurin inhibitors or rituximab, with the main goal of inducing remission and delaying progression to ESKD.
Rituximab has drawn the attention of the scientific community, particularly in the nephrology field, due to its overall safe profile while being successful in minimizing the attack of the immune system on its own cells. Its first use in nephrology was in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, but soon its potential in other entities was investigated further.
Rituximab has now gathered sufficient evidence to be recommended in certain glomerular diseases, as presented in the Kidney Disease Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.5
This paper aims to gather most recent available evidence as to when rituximab should be used in treating different glomerular diseases, what regimens are indicated and what other glomerulopathies may come to benefit from this immunosuppressive in the future. It will also reference adverse events and, although uncommon, serious complications that must be taken into account when prescribing the treatment. Individuals <18 years and kidney transplant recipients will be excluded from this review.
APPLICABILITY IN GLOMERULAR DISEASES
ANCA-associated small vessel vasculitis
This entity was the first glomerular disease to have approved rituximab use as part of its treatment, both in induction and maintenance regimens. Previously, induction treatment encompassed cyclophosphamide and corticosteroids. In a study conducted by Stone JH et al,6 rituximab was non inferior to cyclophosphamide in inducing remission of severe ANCA-associated small vessel vasculitis, results that led to the development of more studies in this field.
Due to high relapse rates following induction with cyclophosphamide, MAINRITSAN7 was developed to assess the efficacy of rituximab as a maintenance treatment, comparatively with azathioprine. Results of this trial showed the group who received anti-CD20 antibody treatment after remission only had 3% major relapse, compared to 29% of the groups treated with azathioprine. RITAZAREM trial analyzed the potential of rituximab to reinduce remission in patients with relapsing disease, irrespective of previous use of rituximab or cyclophosphamide.
Results showed a high rate of remission (>90%) by 4 months8 after re-induction with rituximab. It is also worth mentioning 71% of patients who re-achieved remission had received a low-dose ematúr of corticosteroids (0.5 mg/kg/day).
Following solid evidence, rituximab’s use is now approved as induction therapy in de novo ANCA-associated small vessel ematúria , together with corticosteroids, and should be preferred to cyclophosphamide (also a first line option for induction treatment) in children and ematúria s, pre-menopausal women and men concerned with their fertility, frail elderly, relapsing disease and in proteinase 3 (PR3)-ANCA disease. Cyclophosphamide, on the other hand, is indicated in severe glomerulonephritis with emat creatinine >4 mg/dL. KDIGO guidelines recommend rituximab induction with either 1 g on week 0 and on week 2, or 375 mg/m2 weekly for 4 weeks. Besides, if diffuse alveolar hemorrhage with hypoxemia is ematúri, in patients requiring dialysis or with rapidly increasing emat creatinine, plasma ematúri (PEX) should be considered prior to administering rituximab.5 Aside from these situations, no trial so far, including PEXIVAS,9 demonstrated benefit in the prevalence of ESKD or death rates with PEX therapy in ANCA-associated small vessel ematúria . Also, induction therapy should be discontinued after 3 months in patients that are still dialysisdependent and that show no signs or symptoms of extra-renal disease.5
For maintenance therapy, rituximab alone ought to be preferred instead of combined therapy with azathioprine and corticosteroids, in the following situations: relapsing disease, PR3-ANCA disease, frail elderly, azathioprine allergy and those where glucocorticoid-sparing is especially ematúria . Maintenance therapy should also be ensued in patients with ANCA/anti-glomerular basement membrane disease overlap, given the higher relapse risk.5 The recommended scheme is based on MAINRITSAN trial, administering 500 mg on week 0 and week 2 after complete remission, followed by 500 mg on month 6, 12 and 18.5,7Minimum recommended duration of maintenance therapy is 18 months,5 although optimal duration is still unknown.
Response to rituximab can be monitored based on signs and symptoms, and by monitoring renal function and assessing urinary sediment for microscopic ematúria.
As for re-inducing remission in relapsing disease (Table 1), treatment should be based on RITAZAREM scheme: 1 g after remission induction, and then on month 4, 8, 12 and 16 after first rituximab infusion.
Table 1 Definitions of remission, relapse, steroid-dependent and refractory diseases based on the specific glomerular disease
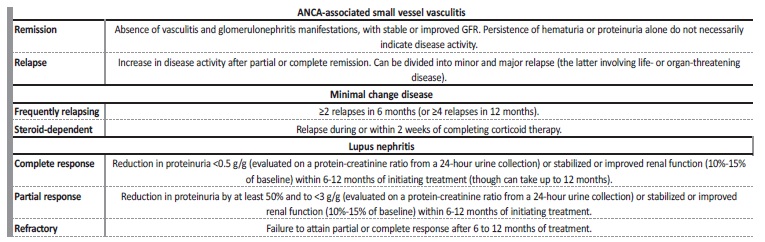
GFR - glomerular filtration rate.
Adapted from: Rovin BH, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1-276.5
Primary membranous nephropathy (MN) MN is one of the many causes for nephrotic syndrome and can lead to ESKD within 10 years. While initial therapy is based on supportive measures, there are cases where immunosuppression is indicated, particularly in high-risk patients (Table 2) and in persistente nephrotic syndrome. The first randomized controlled trial (RCT) developed to analyze rituximab efficiency in MN was GEMRITUX, concluding that despite the monoclonal antibody being as effective at reducing proteinuria as supportive measures, it achieved higher long-term remission rates at 17 months of follow-up.10 The MENTOR study11 compared treatment response of MN patients at high risk of disease progression, after selecting one group to receive rituximab and another cyclosporine. Rituximab was efficient at reducing proteinuria at 12 months and was non-inferior to cyclosporine, with better results than cyclosporine in relation to proteinuria remission at 24 months followup.
Table 2 Criteria to assess risk of progressive loss of kidney function in membranous nephropathy
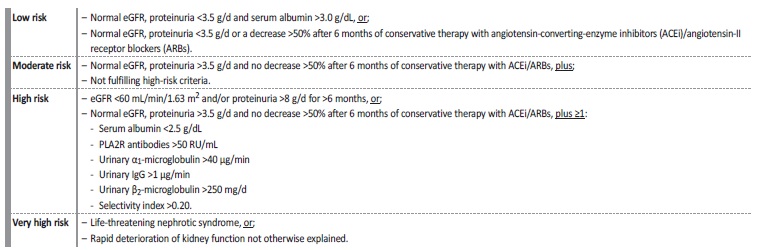
eGFR - estimated glomerular filtration rate; ACEi - angiotensin converting enzyme inhibitor; ARBs - angiotensin-II receptor blockers; PLA2R - phospholipase A2 receptor; RU - relative units; IgG - immunoglobulin G.
Adapted from: Rovin BH, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1-276.5
The third and most recent is STARMEN,12 which compared remission rates after alternating treatment with corticosteroids and cyclophosphamide against sequential treatment with tacrolimus and rituximab. Contrary to what was expected, sequential treatment proved to be less effective in inducing remission. However, it was noted that rituximab increased complete remissions and decreased the number of relapses after tacrolimus tapering. In primary MN KDIGO5 now recommends the use of rituximab in those with moderate to high risk of disease progression. However, there does not seem to be one immunosuppressor that is favored over another.13,14 As stated in a meta-analysis published by Bose B et al,14 apart from cyclophosphamide, there was not sufficient evidence that would recommend one immunosuppressive treatment over the other (between rituximab, mycophenolate mofetil (MMF), calcineurin inhibitors or monotherapy with steroids) in terms of the ability of inducing complete remission and preventing kidney failure.
Despite the latest KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases5 supporting the use of diferente immunosuppressors in MN, based on data mentioned above and knowing there is a higher relapse rate of MN when calcineurin inhibitors are used, as well as the known increased risk of adverse effects with cyclophosphamide use, we advocate the use of rituximab as a first line treatment option for MN in patients at moderate to high risk of disease progression, due to its high efficacy and safety profile.15,16
Regarding regimens, 1 g of rituximab on week 0 and on week 2 (NICE protocol) seem to have better results than 375 mg/m2 at week 0 and week 1 (GEMRITUX protocol). Low-dose regimens (such as GEMRITUX) seem to be less effective than high-dose ones (NICE protocol),16 and indeed the current KDIGO guidelines recommend either the NICE protocol or a scheme of 375 mg/m2 weekly for 4 weeks.5
Immunological monitoring by evaluating anti phospholipase A2 receptor (anti-PLA2R) antibody levels at month 3 and 6 can help guide therapy.5 If after 6 months of treatment there is persistent nephrotic proteinuria and stable estimated glomerular function rate (eGFR), especially if anti-PLA2R antibodies are still positive and there are no anti-rituximab antibodies, repetition of rituximab scheme (1 g on week 0 and on week 2) should be considered.5
Minimal change disease (MCD) in adults
The cornerstone of MCD treatment in adults are glucocorticoids, but adverse effects are well known to be associated with their longterm use. Despite less frequent than other glomerular diseases, MCD can be relapsing and can become steroid-dependent (SD) and steroidresistant (SR) (Table 1).
There have been several observational studies17-19 over the last years that reported rituximab had good efficacy at reducing relapse rates in the long-run in these two sub-entities as well as at reducing proteinuria, with remission induction rates varying from 65% to 100%,5 while also allowing for a reduction in steroid use and other immunosuppressants. Various systematic reviews20,21 have compiled available evidence to date to conclude that while promising, rituximab is still an off-label drug in SD and frequently relapsing (FR) MCD, and its mechanism of action in these disorders is still not completely understood.
Nonetheless, KDIGO guidelines include rituximab as an option in FR and SD MCD, and in patients who have previously received cyclophosphamide therapy or those pretending to avoid this drug, although not as a first-line drug.5 Proposed induction and re-induction post-relapse regimens vary. For the former rituximab can be administered either 1 g on week 0 and on week 2; or 375 mg/m2 weekly for 4 weeks; or 375 mg/m2 once, and repeating dose after 1 week if CD19 cell count ≥5/mm2. In the case of re-induction treatment after relapsing disease, single administration of 1 g or 375 mg/m2 of the anti-CD20 antibody.5
Immunocomplex mediated membranoproliferative glomerulonephritis (MPGN)
MPGN can be divided into two subtypes, complement or immunocomplex mediated, and treatment should primarily be focused on treating underlying disorder. The latter subtype is associated with the production of autoantibodies by B lymphocytes, which led to the hypothesis that rituximab might have a role in interrupting glomerular inflammation, especially in idiopathic MPGN.
Despite some case reports22-24having revealed an important reduction in proteinuria and stabilization of eGFR when rituximab is used in association with corticosteroids, evidence is still scarce and of lowquality, and most data is based on cases of MPGN with cryoglobulinemia.
Nevertheless, and given its known safety profile and side effects associated with cyclophosphamide, it is still an option as a rescue treatment, based on KDIGO guidelines.5 Once patients have been under double immunosuppression for 6 to 12 months with corticosteroids and MMF, and after failure of prior treatment with corticosteroids alone, if no improvement of kidney function and/or proteinuria decrease is only <30%, a repeat biopsy should be considered.5 If histologic findings denote active glomerulonephritis (endocapillary hypercellularity, interstitial inflammation, fibrinoid necrosis, karyorrhexis), rituximab should be considered. If crescents are present, then cyclophosphamide is preferred.
Recommended dosing regimen is 1 g on day 1 and on day 15 and then repeating this scheme on month 6.5
Lupus nephritis (LN)
LN is a concerning complication of systemic lupus erythematosus (SLE), having a deleterious impact on morbidity and mortality. Active LN involves extensive inflammation of the glomerulus and requires immunosuppressive treatment, primarily with corticosteroids, to improve renal outcomes. Second immunosuppressive agent has classically been MMF, cyclophosphamide or tacrolimus. However, rituximab is being studied as an alternative option. LUNAR trial25 is the only RCT available to date evaluating rituximab in initial therapy of LN and did not show improved clinical outcomes when rituximab was used together with MMF and corticosteroids, despite having demonstrated greater reductions in complement and anti-double stranded deoxyribonucleic acid (anti-dsDNA) levels. However, there have been smaller observational studies26,27with promising results in LN class III-V refractory to first-line therapy (Table 1). More trials are underway, which may help define better how the anti-CD20 antibody acts on LN and more precise recommendations for its use.
Based on available evidence, most recommendations designate rituximab as an immunosuppressive option in resistant and relapsing LN class III-V,5,28and recommended regimen is 1 g on day 1, and another infusion of 1 g on day 15.5
Thrombotic microangiopathies (TMA)
Thrombotic thrombocytopenic purpura (TTP)
TTP, a rare disorder associated with microvascular thrombi that cause organ ischemia, can occur as a congenital form or as an acquired (immune-mediated) one. While the latter is associated with the presence of autoantibodies against a disintegrin-like and metalloproteinase with thrombospondin type 1 motif member 13 (ADAMTS13), leading to a decrease in enzyme activity, in the former ADAMTS13 activity is absent and the disease has an earlier onset.29,30
Despite combined therapy of PEX and corticosteroids accounting for a significant reduction in mortality rate, there are still 30%-50% of patients who relapse and others that are refractory to treatment, allowing us to consider it a chronic illness.29,30
Several trials have demonstrated rituximab use had good results in minimizing acquired disease relapse in those who had a history of prior disease recurrency, as well as in those with refractory disease.
Furthermore, some have also showed good results when rituximab was used as initial therapy together with PEX and corticosteroids, with lower relapse rates during follow-up.31-33
In regard to patients on remission, current data indicates that ADAMTS13 activity levels <20% increase the risk of immune TTP relapse. Prophylactic rituximab use in these cases seems to decrease this risk,34-36 but not one scheme has yet been agreed upon.
In the case of an acute episode of TTP, rituximab is now considered as part of a triple-therapy strategy together with PEX and corticosteroids (while caplacizumab should also be initiated early in the course of the disease), according to the 2020 guidelines from International Society on Thrombosis and Hemostasis (ISTH).36 The regimen varies, but the most accepted treatment is 375 mg/m2 weekly for 4 weeks. Rituximab must be administered immediately after plasmapheresis, and at least 24 hours before the next PEX.
Complement mediated atypical hemolytic uremic syndrome (aHUS)
This entity presents itself as a challenging glomerulopathy to the clinician, especially when it comes to its management. Complement mediated aHUS with antifactor H antibodies (anti-CFHAbs) is an even rarer form of the disease. PEX should be initiated as soon as a diagnosis of TMA is made, and complement inhibitory therapy with eculizumab is indicated in adults as soon as TTP is ruled out. Initiation of targeted therapy within 24-48 hours is fundamental to improve renal and overall outcomes.37,38
Despite not existing, yet, solid evidence to the routine use of rituximab, when anti-CFHAbs are present there seems to exist an advantage in its use, controlling antibody formation. Rituximab may become na important therapeutic agent in these cases (not exempting the use of PEX therapy nor eculizumab in the acute setting), with the purpose of inducing remission and avoiding the need for maintenance immunosuppressants.
However, to date this evidence is based on case series only,39-41with no controlled studies available yet. The use of rituximab in aHUS with anti-CFHAbs should be discussed, if possible, with a complement expert.
EVIDENCE IN OTHER GLOMERULOPATHIES
There are other glomerular diseases which may come to benefit from immunosuppression with rituximab, although currently there is no sufficient data to encourage its use. Examples include IgA nephropathy (IgAN), focal segmental glomerulosclerosis (FSGS) and cryoglobulinemic glomerulonephritis.
There have been a few IgAN cases published (mainly crescentic IgAN) that showed improvement of kidney function and a decrease in proteinuria after rituximab use as induction therapy.42,43However, these changes are not consensual and evidence is still lacking to start considering rituximab as a treatment option in IgAN.44
In FSGS, there may be an advantage in the use of rituximab in cases of SD disease, but not in the case of SR disease. Rituximab seems to help induce remission (either complete or partial) and minimize maintenance therapy.18 However, other studies failed to show rituximab’s efficacy in inducing remission45,46and more studies are needed with larger population samples and FSGS cohort patients exclusively, since most have been mixed with MCD patients.
In hepatitis C associated cryoglobulinemic glomerulonephritis and/or vasculitis, rituximab is now known to be effective and should be considered as a first-line immunosuppressant treatment in moderate to severe disease, according to the Italian Study Group of Cryoglobulinemia.47 These recommendations are based on 3 RCT48-50that showed rituximab improved or stabilized of renal function, achieving better rates of clinical remission. Despite not existing a worldwide recommendation of its use in cryoglobulinemic glomerulonephritis and/or vasculitis, we recommend it should be pondered individually.
OTHER CONSIDERATIONS
Immunophenotyping of peripheral blood
Immunophenotyping of peripheral blood searching for CD-19 B-cells may have an interest when assessing the need for a new rituximab administration, especially 90 days after the last infusion, when these cells start to reconstitute. However, there is no scientific evidence that clearly states that we can consistently determine a new rituximab administration based on these cell counts, because the correlation between them and clinical response is not always linear.51,52A study conducted in a population with rheumatoid arthritis successfully predicted clinical relapse by assessing B-lymphocytes and their subsets regeneration every 2 months, and identified that most relapses occurred 4 months after B cell repopulation.53
Despite the lack of guidelines suggesting when these assessments should be done, we would consider immunophenotyping peripheral blood in search for CD19 cells when there is a high suspicion of recurrence of disease activity, based on symptoms and laboratory data suggestive of disease relapse.
Intravenous immunoglobulin
Assessing the levels of immunoglobulin prior to initiating treatment should be done routinely in order to identify those with a higher risk of developing severe infections (elderly, diabetic, prior use of azathioprine, renal impairment and nephrotic syndrome).54 Hypogammaglobulinemia is a relatively common side effect of rituximab (up to 30%), especially in those with prior or concomitant corticosteroid use, prior exposure to cyclophosphamide and reduced baseline levels of IgG. Moreover, prolonged use of rituximab seems to be associated with a higher risk of developing serious infections, although not all studies have found this relation.2,55
Intravenous immunoglobulin should be contemplated in patients with both a history of recurrent infections and IgG levels <500 mg/dL, in order to prevent severe infections. The first administration varies between 0.2 and 0.4 g/kg, and subsequent infusions should happen only 3-4 weeks after the first one, based on IgG levels. We recommend these values be monitored monthly at first.
Perfusion velocity varies depending on the type of immunoglobulin available, so it is advisable to check it with the hospital pharmacy individually. Furthermore, immunoglobulin administration can be associated with side effects, most commonly flu-like symptoms (headache, chills, fever) that can be minimized by slowing down perfusion velocity. Moderate side effects have been described, like hypotension, arrythmias, transfusion-related acute lung injury, and more severely bronchospasm and anaphylaxis.56
SAFETY PROFILE OF RITUXIMAB
Infectious risk and prophylaxis
• Pneumocystis jirovecii pneumonia (PJP): PJP is an opportunistic fungal infection associated with a high mortality risk (from 30% in rheumatoid arthritis to 62% in ANCA vasculitis). Known risk factors include corticosteroid treatment for >3 months with >15 mg/day, additional immunosuppressives, HIV, malignancy and total lymphocyte levels <600 μL.57,58 Despite the fact there is no consensus yet regarding PJP prophylaxis and despite overall incidence being low,57 given the high mortality reported it seems reasonable to consider prophylaxis in all patients receiving rituximab with at least one of the risk factors stated above. P. jirovecci prophylaxis is done with cotrimoxazole 960 mg 3 times per week (if eGFR >30 mL/min/1.73 m2) or 480 mg 3 times per week (when eGFR ≤30 mL/min/1.73 m2). In case cotrimoxazole is contraindicated, other options include dapsone 100 mg daily or atovaquone 750 mg twice daily.57,58Prophylaxis should be kept for at least 6 months following the last rituximab administration, to allow for B-cell regeneration.
• Hepatitis B virus (HBV) reactivation: Rituximab is associated with a higher risk of chronic HBV infection reactivation, even though this risk is lower in autoimmune disorders than in malignant hematological conditions.54 All patients receiving rituximab should be previously screened for HBV serologies. Those with positive HBV surface antigen and/or hepatitis B core antibody should receive HBV reactivation prophylaxis (which should be discussed with an infectious disease expert). Prophylaxis should be administered in the 7 days prior to initiation of anti-CD20 therapy and until one year after the last treatment. It is also advisable to monitor transaminases levels and viral load every 3 months and up to 6 months after completion of treatment, in those with previous HBV infection in order to detect reactivation early.2,59
• Cytomegalovirus (CMV) pneumonia: CMV infection can be na organ-invasive disease and become lethal. Most studies assessing the risk of CMV pneumonia in immunosuppressed patients have been on transplant recipients, currently existing little scientific evidence regarding glomerular diseases. Lim CC et al60 developed an algorithm to reduce CMV infection in glomerular diseases, based on scientific literature concerning risk factors in non-transplant immunosuppressants recipients. They then compared the incidence of CMV disease in this population, undergoing immunosuppressive treatments, with and without the use of the algorithm. Although the incidence of CMV disease was found to be similar prior and after the implementation of the algorithm, in those who were eligible for prophylaxis in the post-implementation group, CMV disease occurred less frequently.60 Optimal cut-off value to initiate pre-emptive treatment varies between laboratories and depends on the risk of developing CMV disease (varying from 1000 copies/mL to 10000 copies/mL, in high and low risk patients, respectively61). We advocate a multidisciplinary approach and discussion with an expert.
• Herpes simplex virus (HSV) and Varicella zoster virus (VZV) reactivation: Rituximab seems to increase the risk of VZV and HSV reactivation, particularly when the patient is receiving additional immunosuppressive drugs.62 Despite the lack of studies of this risk in glomerular disease patients, Tran CT et al recommend vaccination before starting treatment, when possible. Prophylaxis with acyclovir is not yet recommended in these patients, but extrapolating from evidence on malignancies and hematopoietic stem cell transplanted patients, it should be considered on an individual level in all HSV-1 and HSV-2 seropositive patients, with acyclovir 400 mg twice daily (if eGFR ≥25 mL/min/1.73 m2) or 200 mg twice daily (if eGFR <25 mL/min/1.73 m2).63
• Latent tuberculosis reactivation: In light of current knowledge, there is no evidence of increased Mycobacterium tuberculosis reactivation in kidney transplant patients exposed to rituximab. The known risk is of de novo infection.64,65However, such data has not been studied in non-transplanted patients. Nevertheless, and although there is no formal indication for screening, we advocate assessing IGRA to exclude latent tuberculosis and searching for chest x-ray anomalies that may indicate tuberculosis complications. In these patients, we recommend seeking the opinion of an expert to assess the need for treatment with isoniazid.
• Progressive multifocal leukoencephalopathy (PML): The reactivation of John Cunningham (JC) virus leads to the development of neurological symptoms that comprise a rare form of a demyelinating disease. Although rare PML can be fatal, so it is vital that patients be informed of symptoms which may raise the suspicion of the disease, such as visual disturbances, confusion, decline in cognitive capacities, ataxia and paresis.54,66There seems to be a higher risk for patients with SLE and those treated with additional immunosuppressives.2,66Diagnosis is made by identifying JC virus in cerebrospinal fluid and characteristic findings on magnetic resonance imaging.2
Vaccination
Immunization prior to treatment is recommended, since immune response following rituximab treatment may be blunted. Liveattenuated vaccines must be administered up to 4 weeks before the first treatment with rituximab,54,67while inactivated vacines can be administered up to 1 week before. However, if optimal timing to administer the inactivated vaccines cannot be achieved, they should still be administered (even though immunization will only be partial).54 Based on this information, we recommend immunization against Streptococcus pneumoniae, Influenza (annually) and HBV for those at higher risk (HBV-seronegative travelling to or living in endemic regions and those at risk of exposure, such as healthcare workers, intravenous drug users, men who have sex with men).1,66,68
Apart from these, the Portuguese national vaccination plan should be completed (keeping in mind older patients may not be immunized against some pathogens due to changes in the vaccination plan across decades). Some authors defend vaccination against other microorganisms, such as those against Influenza A and B, and against meningitis C.54
After treatment course, in case immunization is needed, there should be a waiting time of at least 6 months since the last rituximab infusion.1,54,67 Furthermore, we recommend evaluation of immunoglobulins and B lymphocytes levels every 3 months until their normalization, to administer vaccination schemes safely.
Regarding SARS-CoV-2 vaccines, it is well established that seroconversion rates are lower with low levels of circulating B cells (such as in patients on anti-CD20 treatment regimens).69 For this reason, we recommend at least two doses of the vaccine are given before beginning of rituximab treatment.69 However, no vaccination scheme should delay immunosuppressive treatment when this is indicated.
Adverse reactions
The most common side effects of rituximab are hypersensitivity reactions, happening mostly during the first infusion, and symptoms may include myalgia (13%-39%) and chills (3%-33%). Fever might occur in up to 56% of cases.2,54 Despite being rare (<12%), severe reactions to rituximab infusion are described and occur mostly with the first administration, 30 to 120 minutes after the start of the treatment.
These reactions manifest themselves with bronchospasm, acute respiratory distress syndrome, hypotension and shock.2,24,70
Other side effects include nausea (8%-23%), diarrhea (10%-17%) and headaches (15%-19%), which can be attenuated with premedication. Hypogammaglobulinemia (1%-58%) and anemia (1%-35%) are some of the hematological changes that can occur with the treatment.2,54,70
Contraindications
Like any drug or treatment, rituximab has its own contraindications. Concerning absolute contraindications, these comprise hypersensitivity to rituximab or to any of the components in its formulation; known allergy to murine products or previous history of severe allergic reactions; severely immunosuppressed patients with neutropenia and/or hypogammaglobulinemia; active infection (acute or chronic); past history of progressive multifocal encephalopathy; pregnancy and lactation; and recently administered live-attenuated vaccines.1,54,66 Chronic infections (HBV, CMV and herpes simplex infection) with a history of frequent reactivations, malignancies or New York Heart Association (NYHA) IV heart failure should be addressed as relative contraindications, and each situation should be considered carefully.1,66
CONCLUSION
The arrival of rituximab in the Nephrology field led to the improvement of immunosuppression treatments in glomerular diseases, providing an enhanced management strategy, a better prognosis and a decrease in side effects of other immunosuppressive drugs.
As described, rituximab has now a formal indication in induction therapy in de novo ANCA-associated small vessel vasculitis as well as in its maintenance and re-inducing remission in relapsing disease. In MN we advocate its use as a first-line option in those with moderate to high risk of disease progression given the adverse effects associated with cyclophosphamide and the higher relapsing rates with calcineurin inhibitors. As for MCD rituximab can still be considered in SD or FR disease, but although promising it is still considered an offlabel drug. Ongoing trials (TURING (EudraCT: 2018-004611-50) and RIFIREINS (NCT03970577)) may clarify the evidence regarding its safety and efficacy in these MCD subtypes. In the case of MPGN the evidence is still scarce and of low-quality, and rituximab should only be considered as a rescue treatment. In resistant and relapsing LN class III-V rituximab may be advantageous, and trials are underway to provide us with more robust evidenc concerning its indication in first line therapy. Moreover, in hepatitis C associated cryoglobulinemic glomerulonephritis rituximab ought to be considered as a first line agent in moderate to severe disease.
In the spectrum of TMA, rituximab should be part of a therapy scheme in acute TTP which also includes caplacizumab, and has also shown good results in prophylaxis of relapsing disease when ADAMTS13 activity is <20%. In aHUS evidence is lacking but there are promising results when anti-CFHAbs are present.
To conclude, despite great advancement in the past decade, further studies are needed to encourage this anti-CD20 therapy more strongly in other glomerular diseases, especially since its use is not without risks. We deem it extremely important to evaluate infectious risk individually prior to starting immunosuppression, with particular attention to PJP, CMV pneumonia and HBV reactivation, as well as assessing vaccination needs.















