INTRODUCTION
Peritoneal dialysis (PD) is a renal replacement therapy that focuses on preserving the quality of life (QoL) of dialysis patients. Compared to hemodialysis (HD), PD allows a greater lifestyle flexibility and independence, better preservation of residual renal function1,2 and a more physiological removal of fluids and toxins, reducing the risk of hemodynamic instability.1 However, PD carries a high infectious risk and requires greater individual responsibility and detailed training to minimize complications.1
Sleep disorders are highly prevalent among PD patients3 and include changes in sleep architecture, sleep disordered breathing (SDB), restless legs syndrome, periodic limb movement disorder and excessive daytime sleepiness.4-7The pathophysiology of sleep disturbances in PD is often multifactorial: chronic fluid retention, uremia,8 negative emotional states (e.g., depression), somatic symptoms, and treatment-related issues (e.g. cycler alarms in patients on APD and abdominal dialysate bulk load during the night exchanges) may also contribute to poor sleep quality in this population.8,9 Nonetheless APD patients appear to have better sleep quality compared to continuous ambulatory PD (CAPD) patients, perhaps due to more effective ultrafiltration and solute clearance.3
Sleep disorders can lead to psychological distress,10 excessive daytime sleepiness, cognitive dysfunction,11 chronic inflammation12 and appear to have significant negative effects on the quality of life as they are often cited as major sources of stress.6,7 Furthermore, there is now a well characterized link between some sleep disorders and cardiovascular (CV) disease: obstructive sleep apnea syndrome has been proven to cause systemic arterial hypertension13,14 and possibly myocardial infarction,15 heart failure16 and sudden death.15-17
Despite the effect of sleep quality on the QoL and morbidity of PD patients, studies addressing the effect of APD on these parameters are lacking. There are some older publications focusing on sleep quality and QoL in PD patients, but they did not differentiate between PD modalities.18,19 The major studies comparing both modalities in respect to QoL and sleep quality did not find significant diferences between groups in any of the analysed parameters.5,20 We aim to compare life and sleep quality between patients on automated PD (APD) and continuous ambulatory PD (CAPD) in a Portuguese cohort.
MATERIAL AND METHODS
We conducted an observational, cross-sectional study in chronic PD patients enrolled in our Dialysis Unit in February 2022. Patients were included only if they had at least six months of dialysis vintage. We excluded patients that stated recent alterations of their sleep pattern or symptoms suggestive of major depressive or generalized anxiety disorders. Patients who switched from CAPD to APD (or vice versa) and patients with bacterial peritonitis in the month prior to our study were also excluded. Demographic data (such as gender, race and age), prior diseases (namely diabetes, hypertension, dyslipidemia, obesity, cardiovascular disease and sleep apnea syndrome) were collected retrospectively from the existing patient records. Mean ambulatory blood pressure measurements from the previous three months, urinary output and PD-related information (transporter type, mean technique Kt/V from the previous three months) were collected at the time of the appointment in which the questionnaires were delivered.
Blood pressure was categorized in: controlled- systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg; uncontrolled - SBP>140 mmHg and/or DBP > 90 mmHg. Each patient received three questionnaires: one regarding QoL (EUROHIS-QOL-8) and two regarding sleep quality (Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS)). EUROHISQOL-8 is a QoL index composed by eight items that evaluate four domains of life (physical, psychic, environmental and social relationships) whereas a higher value corresponds to a higher patient’ evaluation of their QoL.21 PSQI is a self-report questionnaire that assesses sleep quality over a one-month period. This measure consists of 19 individual items, creating seven components that produce one global score ranging from 0 to 21, where lower scores denote a healthier sleep quality.22 A total score of “5” or higher is indicative of poor sleep quality. ESS is an eight-item questionnaire routinely used to assess daytime sleepiness, reflected in a total score ranging from 0 to 24: a score ≥ 11 represents excessive daytime sleepiness, with higher scores associating with a higher sleepiness severity.23
Statistical analysis was performed using IBM-SPSS Statistics v22 and the confidence interval was set on 95%. A p value < 0.05 was considered statistically significant. Mean values and standard deviations were calculated. Comparison of means and frequencies of normally distributed variables were calculated using t-tests and the χ2 test. Pearson’s correlation was used to identify a correlation between different variables. Independent samples t-test was used to compare demographic data, prior diseases, PD-related parameters (residual diuresis, weekly Kt/V, number of exchanges) and the scores of the three questionnaires between APD and CAPD patients.
RESULTS
Our sample was composed of 52 PD patients (63.4% of our total PD population): most were caucasian (96.2%, n=50), males (65.4%, n=34) and had a mean age of 52.7±14.2 years. Undetermined aetiology was the most prevalent cause of ESRD (25.0%, n=15), followed by hypertensive nephropathy (17.3%, n=9), IgA nephropathy (13.5%, n=7) and diabetic nephropathy (9.6%, n=5). There were significant identifiable cardiovascular (CV) risk factors: arterial hypertension (100%, n=82), dyslipidemia (63.5%, n=33), obesity (34.6%, n=18), diabetes (19.2%, n=10), heart failure (19.2%, n=10) and sleep apnea syndrome (SAS) (3.8%, n=2). As a result, 38.5% (n=20) were categorized as having a very-high CV risk, according to the European Society of Cardiology’ CV risk classification.
The prevalence of documented sleep disturbances was 23.1% (n=12) across our sample, with insomnia being the most frequent.
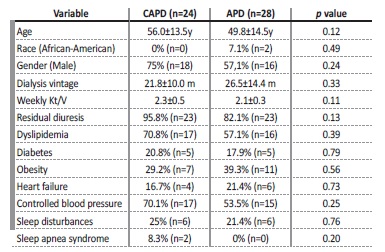
Regarding PD characteristics, our sample had a mean dialysis vintage of 24.3±17.1 months and presented with adequate dialysis efficiency: all patients had a weekly Kt/V (mean from the previous three months) ≥ 1.7, which was reflected in a mean weekly Kt/V of 2.2±0.4, and 61.5% (n=32) had controlled BP. Residual diuresis was present in 88.5 /n=46) of our sample and averaged 1330±380 mL/day. We found a similar distribution between APD (53.8%, n=28) and CAPD (46.2%, n=24) and no significant differences in demographic data, PD-related parameters and baseline comorbidities between both groups (Table 1).
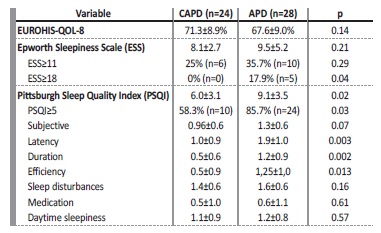
Table 1: Comparison between CAPD and APD patients regarding demographic data, PD-related parameters and baseline comorbidities.
y: years; m: months
Mean QoL index was 69.9±9.3% and was similar between APD and CAPD patients (Table 2). Globally, no correlation was found between patient’s perception of life quality and age, gender, dialysis vintage, PD-related parameters (number of peritoneal exchanges, weekly Kt/V) or the presence of comorbidities (e.g. heart failure, obesity, diabetes or previous sleep disturbances). However, we found an inverse correlation between the EUROHIS-QOL-8 score and the score of both sleep’ quality questionnaires (ESS (Pearson coefficient (r) = -0.39, p=0.004) and the PSQI (r=-0.35, p=0.011)).
Regarding sleep quality, the mean ESS was 8.8±4.2 and the prevalence of excessive daytime sleepiness (ESS≥11) was 26.9% (n=14). Both the total score and the relative frequency of pathological sleepiness were statistically similar between both groups (Table 2). However, the prevalence of severe daytime sleepiness (ESS≥18) was substantially higher in APD patients (17.9% vs 0%, p=0.04). ESS was also higher in patients with previously documented sleep disturbances (11.7±4.6 vs 8.0±3.8, p=0.006) and those with heart failure (11.1±4.3 vs 8.2±4.2, p=0.06).
The mean PSQI score was 7.6±3.6 and the global relative frequency of poor sleep quality (PSQI≥5) was 73.1% (n=34). Both the total PSQI and the frequency of patients with poor sleep quality were significantly higher among APD patients (Table 2). The PSQI score positively correlated with the ESS (r= 0.44, p=0.001) and with residual diuresis (r= 045, p=0.001) and was higher among patients with sleep disturbances (9.8±3.6 vs 7.0±3.4, p=0.014). Furthermore, we found a direct link between sleep quality and blood pressure control: patients with uncontrolled hypertension had higher PSQI (10.5±3.7 vs 5.9±2.2, p<0.0001) and higher scores on the ESS (10.9±4 . vs 7.6±3.6, p=0.005) compared to patients with controlled hypertension. A cut-off value for PSQI superior to 6 was found to be predictive of a higher risk of uncontrolled blood pressure (Area under the curve 0.86, sensibility 95% and specificity 72%).
In our series, the PSQI and the ESS were similar regardless of age, gender, obesity and PD-related parameters (PD efficiency, number of peritoneal exchanges, dialysis vintage).
DISCUSSION
We initially hypothesized that APD patients would have worse sleep quality than CAPD patients since this modality is performed during the night with the assistance of a mechanical device that may interfere with patients’ sleep. On the other hand, we expected a better QoL index in APD patients, since it allows for a more flexible daily Schedule than its respective counterpart. In our series, we found no diferences between PD modalities regarding QoL, but found that APD patients had worse global sleep quality than CAPD patients.
Sleep quality is widely accepted as a major determinant of the QoL of dialysis patients.5,24,25 Iliescu et al, in a study of 89 HD patients, showed that depression and other health-related QoL measures were associated with patient perception of sleep quality.24 In this study, sleep quality correlated with QoL and was a significant independente predictor of the mental and physical components scores of the QoL questionnaire. Sleep disturbances are highly prevalent among PD patients, with several cross-sectional studies reporting that the prevalence of sleep disorders in this population may be as high as 70%.3-5
In 2008, Eryavuz N et al conducted a pioneer study with 102 dialysis patients (52 HD and 50 PD patients) which aimed to compare sleep quality between both groups. Although the prevalence of poor sleep quality was higher in the HD population (88.5% vs 79%), no statistical difference was found. Another study, designed by Guney I et al, compared APD and ACPD regarding health-related QoL, depression, and sleep quality. Although total sleep quality scores tended to be higher in CAPD patients than in APD patients there were no significant diferences between groups in any of the studied parameters. These results were limited by the relatively small population and the mismatch between groups regarding demographical and clinical characteristics.5
In our study, we found that the prevalence of poor sleep quality (PSQI≥5) was 73.1% across our sample. APD patients had a significantly worse sleep quality when compared to CAPD patients. Both the total PSQI (91±3.5 vs 6.0±3.1, p=0.02) and the relative frequency of poor sleep quality (85.7% vs 58.3%, p=0.03) were higher in the APD group.
Despite these results, the prevalence of pathological daytime sleepiness (ESS≥11) was similar between both groups, which may be explained by the fact that excessive daytime sleepiness is often preceded by chronic and sustained poor sleep quality. In our series, we found a significant association between sleep quality and the prevalence of uncontrolled hypertension, highlighting the detrimental effects of poor sleep quality in blood pressure control. The volume of residual diuresis was also an important contributor for a worse perception of global sleep quality among our sample. This is probably due to the fact that most of our sample was comprised by patients with residual diuresis, which are frequently treated with high-dose diuretics, increasing the risk of nocturia and worse sleep quality.
Most studies comparing the QoL between APD and CAPD patients evaluated health-related QoL. De Wit et al27 conducted a Dutch study with 96 PD patients, whereas mental health was found to be better in APD patients. However, no difference was found regarding the physical aspects of the questionnaire. Bro et al had similar results, as no difference was observed between CAPD and APD patients in terms of health-related QoL parameters. Instead of health-related QoL, we evaluated composed QoL (physic, psychic, environmental and social relationships). Nonetheless, our results align with the current existing literature: APD (67.6%±9.0%) and CAPD patients (71.3%±8.9%) had similar scores on the QoL scale (p=0.14). Most importantly, we found a significant correlation between life and sleep quality, which corroborates the pivotal role of quality sleep in the QoL of PD patients.
Our study has some limitations. Firstly, we have a relatively small study sample, especially when comparing to our overall PD population, which was composed of 82 patients. Most patients that were not included did not meet the inclusion criteria and/or exclusion criteria and some patients refused to fill the questionnaires for being so extensive.
Secondly, we evaluated composed QoL and not the health-related QoL, which is more specific when evaluating health-related parameters.
However, by evaluating composed QoL we achieved a more holistic view of the patient, consequently strengthening the results of our study. In our series, patients on APD had worse global sleep quality and higher prevalence of severe daytime sleepiness than CAPD patients. The assessment of the quality of life showed no difference between groups; however, there was a significant correlation between sleep quality and QoL. Furthermore, we found a direct link between sleep quality and blood pressure control, with worse sleep quality parameters associated with a higher risk of uncontrolled hypertension. Future studies this subject are required to investigate the causes for the attained difference in sleep quality between ACPD and APD patients.26