INTRODUCTION
According to the Registry of the Portuguese Society of Nephrology, the number of patients admitted annually for hemodialysis (HD) has increased in the last two decades and the average age has gradually increased.1 This older population has a higher rate of comorbidities, a heavier symptom burden and lower survival rates.2 From the international literature, we know that patients with end-stage kidney disease (ESKD) are exposed to more aggressive treatments than patients suffering from cancer or other chronic diseases, although it is known that their annual mortality rates are comparable.3,4 In fact, patients with advanced chronic kidney disease (CKD) tend to spend more days in the hospital or intensive care unit in their last days of life than patients with other serious diseases.4 Studies have shown that more intensive patterns of end-of-life (EOL) care are mainly focused on prolonging life rather than patient comfort, quality-of-life, and satisfaction.4 Even considering the progressive deterioration of functional status and expected death, most of these patients still have unmet needs related to palliative care, especially symptom control or advance care planning.3,5 According to the literature on EOL care, patients with end-stage CKD are less likely to seek palliative care advice or have a do-not-resuscitate order than patients with dementia or cancer.6 Palliative care is not as widely available for ESKD patients. Moreover, end-of-life care (a subset of palliative care) is poorly known among Portuguese physicians, especially nephrologists.3,5,7,8Our study aims to describe the practices of end-of-life (EOL) care in HD patients in NephroCare clinics in several cities in Portugal.
MATERIAL AND METHODS
This report was conducted according to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) reporting.
The Portuguese NephroCare Review Board approved this study and granted a waiver of informed consent because all study subjects were deceased.
We prospectively recruited patients 18 years or older on maintenance HD from six dialysis clinics in Portugal (Barreiro, Lumiar, Santarém, Setúbal, Venda Nova, Feira), over a 12-month period, from October 2020 to September 2021.
Patients were excluded if they initiated HD less than 30 days prior to recruitment, if they were converted to peritoneal dialysis before the end of the study, or if they had incomplete data.
During the defined period, we collected responses from the responsible nephrologists to the surprise question (SQ) “Would I be surprised if this patient died in the next 6 months?” Responses to SQ were either ‘Yes’ or ‘No’. Responses were collected at two time points at baseline.
All included patients were followed up until death or September 30, 2021. Data for this study were obtained retrospectively from the NephroCare electronic health database (EUCLID©) and from postdischarge clinical information available in the patient’s medical record at the HD clinic. Data were systematically reviewed by a physician from each of the participating dialysis unit.
At baseline, we recorded demographic information, renal failure etiology, comorbidities, Charlson comorbidity index, age-adjusted Charlson index, and dialysis vintage.
We designed a proforma on various aspects of EOL care. We reviewed: (i) causes of death; (ii) location where patients died; (iii) provision of specialized palliative care services for patients who died during the follow-up period; (iv) documented advance directive; (v) hospitalizations in the three months before death, including details of hospitalizations (causes, number of hospitalizations and length of stay); (vi) HD vascular access procedures in the last month of life (vii) death in hospital (rather than death in hospice). The last three variables reflect the intensity of EOL treatment intensity.
Palliative care services were defined as palliative care specialists working in the hospital or community.
The place of death was divided into hospital, home, nursing home, dialysis clinic, special palliative care unit, and others. HD withdrawal was defined as discontinuation of dialysis after an active decision by the patient, family, health care proxy, or treatment team that was documented in the patient’s electronic medical record.
Death after dialysis discontinuation was defined as a death that occurred > 24 hours after HD withdrawal.
Dialysis abandonment was defined as failure to resume HD sessions without prior discussion with a clinical team member.
The existence of an advance directive included: (i) a copy of the advance directive in the patient’s records; or (ii) documented confirmation in the patient’s records that the person has an advance directive that states his or her wishes. The circumstances for the completion of an advance directive were sought in the medical records.
RESULTS
Of all 1265 patients treated with HD in the six NephroCare dialysis units, 159 patients (12.4%) died within the study period.
One patient was excluded because of missing data, resulting in a final sample of 158 patients. Patient characteristics are shown in Table 1. Overall, the mean age of patients was 76 years (25% of patients were older than 85 years). The majority of the cohort were men (68.3%) and Caucasian (91.8%). The most common comorbidities included diabetes mellitus (49.4%), congestive heart failure (40.5%) and peripheral vascular disease (40.5%). Nearly one quarter (24.7%) of patients who died had cancer. Diabetic kidney disease was the most common cause of CKD (36.7%), followed by hypertension (18.4%). The vintage of dialysis treatment was 89 (2-963) months. Patients had a high comorbidity index (age-adapted Charlson index 13 ± 3). For the nephrologists, death was expected in more than half of the cases (in 82 cases, the nephrologists answered “No” to the surprise question - “I would not be surprised if this patient dies in 6 months”).
Table 1: Demographic and clinical characteristics of the cohort
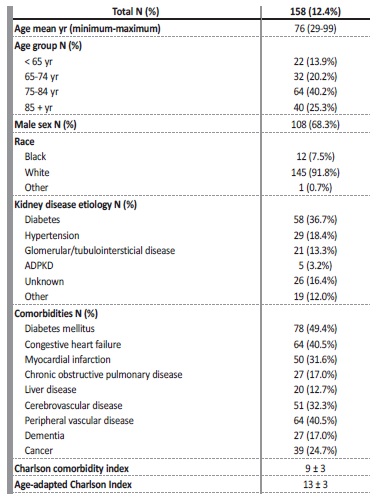
ADPKD - autosomal dominant polycystic kidney disease; yr - years
In terms of treatment intensity, 73 patients (46.2%) were hospitalized at least once in the last three months of life, resulting in a total of 125 hospitalizations.
The average frequency of hospitalizations was 169 admissions per patient in the last three months of life (shown in Table 2). The average length of stay in the last three months of life was 17.2 days (SD 20.5).
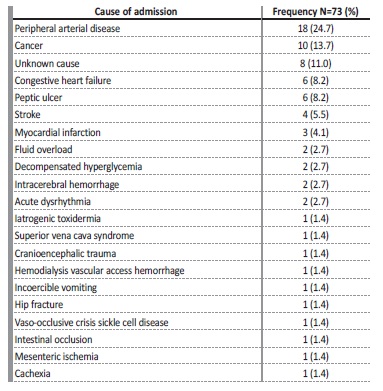
Sixteen patients (21.9%) had hospitalizations totalling more than two weeks of duration in the last 3 months before death. Regarding the cause of hospitalizations (shown in Tables 3 and 4), non-infectious causes were more common and accounted for 58.4% (73/125) of admissions. Peripheral arterial disease was the most common cause (24.7%), followed by cancer (24%) and decompensated heart failure (11%). In 11% of cases, the cause of hospitalization was unknown to lack of access to post-discharge clinical information.
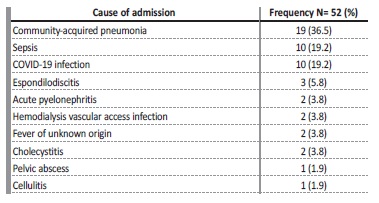
Among the infectious causes of hospital admissions, communityacquired pneumonia (n=19, 36.5%) was the most common cause, followed by COVID-19 and other infections with the same prevalence
values (n=10; 19.2%).
Fourteen patients underwent endovascular procedures on their HD vascular access in the last month of life. In eight of these patients (57.1%), the nephrologists had answered “No” to the surprise question.
Regarding the place of death, 118 patients died in the hospital (74.2%). In 81 of these patients (68.6%), the answer to the surprise question was “No”.
Thirty-six patients died at home or in a nursing home (22.8%) and two died in the dialysis unit (of sudden death and cachexia, respectively). Of those who withdrew or abandoned dialysis (n = 7), five died at home or in the nursing home.
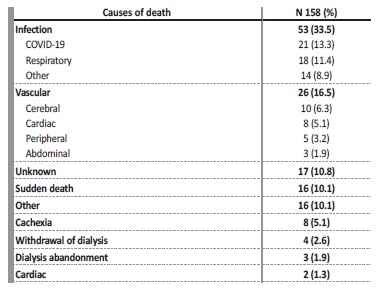
Regarding HD treatments, most patients performed their last dialysis session an average of 5 ± 9 days before death. The causes of death are shown in Table 5. Infectious causes were the most prevalent (35.4%), with COVID-19 accounting for 13.3% of deaths.
Death due to atherosclerotic events was also relevant (16.5%) and is similar to what is described in other ESKD cohorts. Deaths attributed to HD withdrawal or abandonment are less frequent than in
Anglo-Saxon studies, probably because it is not a usual practice in Portugal.
In our cohort, only two patients had an advance directive in our registries. Seventeen had access to palliative care (considering intervention by a differentiated palliative care team). Of these, five had cancer, four had planned HD withdrawal and three had cachexia/frailty. Eighteen patients had opioids prescribed in the last month of life. Of these, eight had cancer, three had a planned HD withdrawal and one was due to osteoarthritis. The remaining causes for opioid prescription were not stated.
DISCUSSION
Our cohort seems to be representative of the Portuguese population on dialysis according to the Portuguese Society of Nephrology Registry1 in terms of mean age, most common comorbidities (such as diabetes mellitus, congestive heart failure or peripheral vascular disease), as well in kidney disease etiology. Our patients presented an elevated vintage in dialysis (more than seven years) and high comorbidity index. Not surprisingly, more than two-thirds of them were expected to die in less than 6 months. Despite a seemingly a poor prognosis, the vast majority of patients received intensive care treatment in the last 90 days of life, resulting in hospitalizations, vascular access interventions or dialysis sessions in the last week of life.
In the last three months of life, patients spent an average of 17 days in the hospital, in addition to complying with a typical Schedule provided for chronic dialysis treatment of four hours three times a week for 90 days), adding up to a total of 21 days of dialysis treatment.
Thus, at least 23.3 % of the patients´ time during the three months before death was spent on dialysis and the burden of necessary hospitalizations related to CKD and all other comorbidities became evident.
The authors underline that not a single patient received hospice services, only two patients had advance care directives and only about 10% received palliative interventions or opioid prescriptions (mainly in an oncology context) reflecting the inadequate application of palliative care principles and poor implementation of EOL care standards in clinical practice. Reasons for the low involvement of palliative care could be the prognostic uncertainty, lack of shared decision making and lack of training or discomfort of nephrologists regarding EOL issues, but, above all, a poor palliative care network or nephrologists’ awareness of it use.11
Few patients had a planned dialysis withdrawal, and only five patients had the option of dying at home. This is in contrast to data from the United States, where dialysis withdrawal is a leading cause of death.12 It also contrasts with data from the United Kingdom, where hospices and the patient’s own home are the main places of death for these patients.13 These results may be explained by the limited number of beds in palliative services or by nephrologists’ unawareness of how to access these services.14
Given the time period of our data collection, it is inevitable to address the impact of the COVID-19 pandemics on mortality. This infection was directly responsible for one third of all deaths, which is consistent with other reports in a cohort of dialysis patients from Switzerland, where 34% of deaths were attributable to COVID-19 during the same period.15 Population-based studies from the European Renal Association registry suggest a high mortality in the dialysis population with rates ranging from 29% to 41% (compared with the average of 11.7% in the general European Union population), mainly related to age and frailty,16,17 but a detailed analysis of this issue is beyond the scope of this article.
Regarding prognosis, as described in other studies, most deaths occur after an acute clinical deterioration requiring hospitalization.
The introduction of palliative care, particularly symptom control or advance care planning, often occurs late, highlighting the importance of early recognition of patients with ESKD on a trajectory of decline who could benefit from advance care planning discussions.18
The use of the SQ is described as 52% vs 76% in other cohorts,19 but its accuracy reflects palliative care education. Its use is more important in recognizing the high likelihood of death in these patients, which should ultimately lead to a change in attitude towards integration of palliative care in the usual care of these patients. The question of whether nephrologists perceive red flags, such as patient’s advanced age or frailty, to ask themselves the SQ and identify patients suitable for palliative care remains unanswered. Identifying the pattern of illness trajectory and the occurrence of sentinel events provides opportunities to address goals of care, patient’s symptoms, or psychosocial and spiritual support to patients and families and ethical issues in dialysis decision making.5,7
Planned access to palliative care is associated with fewer emergency dialyses and less aggressive procedures.8 Our data confirm that palliative care is rarely offered to HD patients, and when it is, it is usually in the context of oncologic treatment. Disturbingly, 5% of mortality was due to cachexia and some died in the dialysis facility, without a planned strategy although several studies confirm that cachexia is a strong predictor of mortality.20 A patient dying of cachexia in a dialysis facility demonstrates the importance of addressing advance care planning, withdrawal of futile medications and education about lifelong care. This serious matter desserves reflection, which is why we consider our work to be relevant in current clinical practice. Nevertheless, literature in ESKD reports that patients experience a variety of symptoms (pruritus, fatigue, pain, sleep disturbances, nausea, muscle cramps, restless legs, anorexia, depression, and dyspnea) that affect their quality of life and have a considerable mortality rate. Therefore, it is urgent to discuss the prognosis in order to plan for EOL care, considering one’s personal and family values.11 In our study, symptom assessment was not performed, however pain control is worth mentioning.
In our cohort, only 11% of patients had a registry of opioid use in their last month of life, mainly in an oncology context. Opioid use is not synonymous with pain control. Pain assessment and medication appropriateness should be reviewed regularly in any context.
In fact, uncontrolled pain should be viewed with caution as pain is often underestimated, especially in patients with dementia, even by relatives.6
Regarding shared decision making, current EOL treatment practices may not reflect patients’ wishes.18 Indeed, little is known about our patients´ preferences and perhaps we do not ask enough about their wishes and perspectives when it comes to EOL issues. According to the literature, 70% of the Australian general population say they prefer to die at home, and about 50% die in an acute hospital setting.2 In the same study, 91% of dialysis patients who were still in the dialysis program died in the hospital.2 Our results are consistent with those from Australia. Some strategies have been proposed to improve advance care planning, including early introduction of EOL care discussions in the nephrologist-patient relationship.7 It is estimated that only 30% of dialysis patients complete written advance directives, although 80% of patients discuss their wishes regarding EOL care with their families.21
Dementia was diagnosed in 17% of our patients, raising awareness of the high prevalence of cognitive impairment in CKD patients22 and challenges associated with it. This should lead to family conferences with EOL discussions in the “best interest of the patient” with those who know them best.23 Because advance care planning is a communication process between patients, families, and health care providers, it is important to improve communication skills and encourage all members of the health care team to participate in the discussion.7,21 Fortunately, the importance of EOL care is increasingly recognized by patients with ESKD and clinicians.10 Indeed, the development of guidelines and collaboration between nephrologists and physicians trained in palliative care should be improved to involve patients and family members in this decision-making process, and to strengthen the relationship between the patient and the nephrologist.18,24
A final issue that should be explored is the quality of dying. Some studies show that symptom management in the hospital can be poor in acute situations and is often recognized late.2 Some tools have been proposed to measure the quality of dying.
Cohen and colleagues proposed the Dialysis Quality of Dying Apgar, but it has not been extensively validated and does not take into account the most important determinants of a patient’s quality of dying, namely patient’s and family’s perspectives.25,26 Certain domains are consistently reported as important in measuring quality of death such as the recognition of dying, symptom documentation, aggressive interventions in the last week of life, documentation of not for resuscitation orders, conversations with the patient and family, assessment of spiritual or religious needs and anticipatory prescriptions and bereavement.2 Our cohort seems to fail on most of these points: although information is limited, we can state that the dying process was poorly recognized, as many patients were exposed to intensive interventions in their last days of life.
Our study has some strengths: it is a prospective, multicentre study, with an important number of patients, representative of the Portuguese population observed in this setting. In addition, to our knowledge, it is the first Portuguese study on this topic.
Nonetheless, we would also like to point out its limitations. First of all, our study is a descriptive study that aims to highlight the importance of the topic. The available information was sparse and limited to the official registries of dialysis units. We lack data on assessment and palliation of EOL symptoms, and we do not have a formal charge to assess frailty. We also do not have information on the reasons why there were so few Palliative Care referrals (possibly due to the lack of a palliative care network and/or low recognition of EOL). Our data were collected during the COVID-19 pandemic. This introduces an unpredictable bias, as the level of care to dialysis patients, access to primary care, and access to hospital care was, most of the times, diminished or at least modified. Therefore, EOL planning was, almost certainly lower than in a non-pandemic context. The nature of palliative care between communities is also very heterogeneous, so the reality may be different in diverse settings.
Future studies should seek to clarify these unanswered questions, make new research hypothesis and explore variable associations, namely the need of further nephrologists training in palliative care, the most adequate model to approach EOL care in Portuguese HD patients in its cultural and particular context.9
CONCLUSION
The Nephrology community is dealing with a growing number of dialysis patients who are elderly and frail, and whose symptom burden is even greater than that of cancer patients. Death is an expected event in this population, although palliative care is not a reality in our ESKD patients. Our study contributes to the understanding of how patients with kidney disease die in the acute hospital setting when palliative care is underutilized. This article should raise awareness of the importance of changing this situation integrating knowledge of palliative care into our routine care. This may be the only way to improve quality of life, to successfully manage symptoms, to recognize EOL and align patient preferences with goals of care and avoid invasive interventions that do nothing but prolong suffering rather than living or preparing for a good death.