CLINICAL PRESENTATION
We report the case of a 46-year-old woman referred to nephrology consult for non-nephrotic proteinuria and hematuria. Her medical history is relevant for type 1 diabetes mellitus (diagnosed at 30 years of age), autoimmune hepatitis (diagnosed at 32 years of age, triggered by hepatitis A virus), systemic lupus erythematosus (diagnosed at 33 years of age; presenting with photosensitivity complaints, autoimmune hemolytic anemia, positive ANA test and low C4 levels), and autoimmune thyroiditis. She has peripheral neuropathy and no evidence of diabetic retinopathy (last ophthalmologic work up carried out one year ago).
She is on ramipril 2.5 mg once daily, levothyroxine 1.25 mg once daily, pregabalin 150 mg twice daily, ursodeoxycholic acid twice daily, and insulin scheme. Prednisolone therapy was stopped 2 years ago, and hydroxychloroquine was stopped due to retinal toxicity.
At first consult, she denied any constitutional symptoms, de novo arthritis, or urinary symptoms. There was no recent change in medication or over-the-counter medication intake. Her bloodwork showed normal hemogram, normal renal function, appropriate glycemic control (glycated hemoglobin of 7%), normal erythrocyte sedimentation rate (32 mm/h). She has increasing albuminuria (100 mg/g creatinine 5 years ago, and at consult 727 mg on a 24-hour urine collection), and urinalysis with de novo hematuria (10 erythrocytes per high power field (HPF)), and leukocyturia (8 leukocytes per HPF). The immunologic study is relevant for increased serum free light chain ratio (3.07; kappa predominance) without M protein detection on serum protein electrophoresis and immunofixation, normal C3 and C4 complement levels, negative ANA and anti-double-stranded DNA.
The patient family history is significant for kidney disease of undetermined etiology: her father required dialysis and ultimately renal transplant. There is no family history of diabetes, autoimmune disease or hearing impairment. Given the uncertainty of the diagnosis, a renal biopsy was performed.
QUESTIONS
1. What are the most likely diagnoses, considering the clinical history and presentation?
2. What is the final diagnosis, considering the histopathological findings?
3. How would you treat this patient?
4. What is our patient’s prognosis?
ANSWERS
1. What are the most likely diagnoses, considering the clinical history
and presentation?
We present the case of a 46-year-old woman with history of systemic lupus erythematosus (SLE) and type 1 diabetes mellitus presenting with sub-nephrotic proteinuria and active urine sediment.
As established, about 50% of the patients with SLE develop lupus nephritis (LN) during the disease, typically defined by the presence of proteinuria, hematuria, and/or increased serum creatinine.1 The absence of immunologic findings often associated with active LN, namely the presence of anti-double-stranded-DNA antibody titers and low complement C3 and C4 levels, makes this diagnosis less obvious.
Despite having no signs of diabetic retinopathy, the presence of increasing albuminuria (100 mg/g creatinine 5 years ago, and at current consult approximately 700 mg per day), in a patient with a 15-year history of diabetes mellitus, is concerning for diabetic nephropathy (DN). DN affects thirty to 40 percent of patients with diabetes mellitus, typically 15 to 20 years after the diagnosis in type 1 diabetes, and poor glycemic control and increasing duration of DM are risk factos that increase the risk of DN.2,3
Increased serum free light chain ratio makes us suspect a monoclonal disease. Although the patient has no anemia, hypercalcemia, bone lesions, or kidney impairment that might raise suspicion for malignant monoclonal gammopathy, increased serum free light chain and albumin predominant proteinuria could also raise the possibility for monoclonal gammopathy of renal significance. Definite diagnosis requires histologic assessment for light chain selective IF fixation based on the distinction according to the organization of deposits on electron microscopy.
IgA nephropathy should also be considered in a patient with liver disease and active urine sediment, given its prevalence as the most common glomerulonephritis. Alport syndrome could be considered given the family history of renal disease in a first-degree member, but neither the patient nor her father had hearing impairment making this diagnosis unlikely.
2. What is the final diagnosis, considering the histopathological findings?
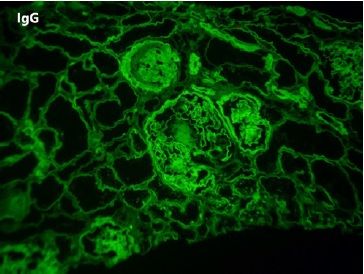
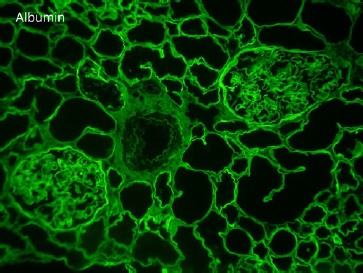
On renal biopsy, two of 14 glomeruli were globally sclerotic. The remainder were hypertrophied with mesangial proliferation forming nodules (Figs. 1 and 3). There was diffuse thickening of glomerular basement membranes, one glomerulus had a lipidic drop in the Bowman capsule (Fig. 2). Moderate tubular atrophy and interstitial fibrosis occupied approximately 10% of the órtex and moderately severe arteriolar hyalinosis (Fig. 3) and intimal fibrosis, causing a 50% lumen reduction. Congo red stain for amyloid was negative. Immunofluorescence showed 1+ linear staining for IgG (Fig. 4) and albumin (Fig. 5) involving GBM and TBM. Staining for IgA, IgM, C3, C1q, fibrin, and κ and λ was negative.
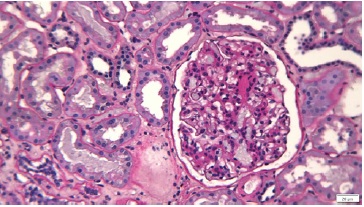
Figure 1 Periodic-acid Schiff. Enlarged glomerulus showing areas of diffuse mesangial hypercellularity and sclerosis
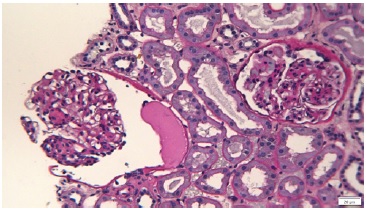
Figure 2 Periodic-acid Schiff. Glomeruli showing diffuse thickening of glomerular basement membranes, one with a lipidic drop in the Bowman’s capsule.
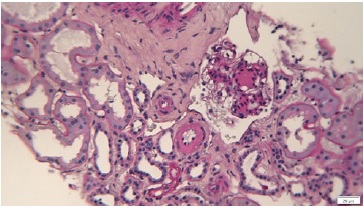
Figure 3 Periodic-acid Schiff. Glomeruli with Kimmelstiel-Wilson nodule and interstitium showing moderate tubular atrophy with interstitial fibrosis occupying approximately 10% of the cortex and moderately severe arteriolar hyalinosis and intimal fibrosis, causing a 50% lumen reduction.
This histopathological pattern, known as nodular glomerulosclerosis, is associated with several diseases namely diabetic nephropathy, chronic membranoproliferative glomerulosclerosis, and dysproteinemia-related glomerulopathies. Less frequent entities include idiopathic nodular glomerulosclerosis (associated with smoking) and pathologic changes related to chronic hypertension, chronic hypoxic and ischemic conditions (eg, renal stenosis).4
In this case, the combination of glomerular basement membrane thickening staining positive for IgG and albumin, the presence of Kimmelstiel-Wilson nodules, the capsular drop, and the arteriolar hyalinosis are characteristic for diabetic nephropathy. This patient arteriolar hyalinosis (Fig. 3) and intimal fibrosis, causing a 50% lumen reduction. Congo red stain for amyloid was negative. Immunofluorescence showed 1+ linear staining for IgG (Fig. 4) and albumin (Fig. 5) involving GBM and TBM. Staining for IgA, IgM, C3, C1q, fibrin, and κ and λ was negative.
This histopathological pattern, known as nodular glomerulosclerosis, is associated with several diseases namely diabetic nephropathy, chronic membranoproliferative glomerulosclerosis, and dysproteinemia-related glomerulopathies. Less frequent entities include idiopathic nodular glomerulosclerosis (associated with smoking) and pathologic changes related to chronic hypertension, chronic hypoxic and ischemic conditions (eg, renal stenosis).4
In this case, the combination of glomerular basement membrane thickening staining positive for IgG and albumin, the presence of Kimmelstiel-Wilson nodules, the capsular drop, and the arteriolar hyalinosis are characteristic for diabetic nephropathy. This patient with a history of diabetes mellitus, non-smoker, lack of long-standing hypertension, and the absence of other immune deposits by immunofluorescence has a typical histology of class III diabetic nephropathy. The urine sediment in diabetic kidney disease is usually bland, but several studies have shown that microscopic hematúria can occur in patients with biopsy proven DN with a rate of 15% to 35%.5-7
3. How would you treat this patient?
The treatment should focus on cardiovascular risk reduction with intensive glucose, blood pressure and lipid control, salt restriction, proteinuria reduction, and active lifestyle implementation.7
Intensive glycemic control in T1DM allows for a significant reduction in the risk of developing proteinuria, an independente risk factor to CKD progression. An individualized HbA1c goal is recommended.8,9BP control also has benefits on any DM-related complication and goals of 130/80 have been suggested but should also be individualized.10
Renin-angiotensin-aldosterone blocking medication was also demonstrated to be effective in delaying the progression of kidney disease, independent of their effect on BP, and should be started in diabetic patients with proteinuria, to the highest approval dose.8
Newer drugs, like the third-generation mineralocorticoid receptor antagonist, finerenone, and sodium-glucose co-transporter 2 inhibitors (iSGLT2) can further improve proteinuria control. Studies are needed on the use of iSGLT2 on type 1 diabetes mellitus with possible concern for increased risk for euglycemic diabetic ketoacidosis.11,12
4. What is our patient’s prognosis?
In our patient, investigating the renal pathology not only contributed to understanding the etiology of proteinuria but also to exclude a superimposed glomerular disease, which would require specific therapy.
Diabetes is the leading cause of chronic kidney disease worldwide and an important proportion of people with diabetic kidney disease will have progressive loss of kidney function and will develop end-stage kidney disease. The strongest risk factor for the progression of kidney disease is the presence of increased albuminuria.
Data regarding the pathologic features with prognostic utility in DN patients are limited. Nevertheless, some studies have shown some association between progression of disease and reduced renal function with severity of mesangial sclerosis and global glomerulosclerosis, but more strongly with interstitial fibrosis.7,13Some authors find na association between the presence of class III diabetic nephropathy and a 5-year renal survival of 36%-39%.13,14
We should consider strategies to control hypertension and cardiovascular disease with optimization of the metabolic control and surveillance of her SLE disease. Also, we should be attentive to the risk of lupus nephritis emergence which would require additional immunosuppression.