INTRODUCTION
Encapsulating peritoneal sclerosis (EPS) is a relatively rare but severe complication of peritoneal dialysis (PD).1-3 The International Society defines EPS as “a syndrome in which adhesions of a diffusely thickened peritoneum cause repetitive and intermittent signs and symptoms of intestinal obstruction”.3 The diagnosis is suggested by abdominal pain, weight loss, nausea, vomiting, anorexia, opaque dialysis effluent, and reduced ultrafiltration. Sometimes only direct abdominal observation in the suggestive clinical picture may lead to the diagnosis.4 EPS is associated with technical failure, significant morbidity, and high mortality.1,5EPS incidence in PD patients ranges from 0.7% to 3.3% and increases with PD duration.1,6,7 The reported mortality range is 25%-55%, especially during the first year after diagnosis, and increases with the time on PD.1,2,8
Different approaches have been tested with controversial results, including total parenteral nutrition (TPN), surgical enterolysis, and pharmacotherapies (corticoids, tamoxifen, and other immunosuppressants). While some retrospective studies showed improved survival of critically ill patients treated with tamoxifen,9 others defend surgical treatment as the first option in EPS management.10 Nowadays EPS treatment remains a big challenge for PD clinicians.9
CASE REPORT
We describe the case of a 36-year-old woman with chronic kidney disease (CKD) secondary to lupus nephritis. She experienced preemptive living kidney donation with early graft failure due to vascular complications. The patient had been maintained on continuous ambulatory peritoneal dialysis (CAPD) without peritonitis episodes, exit-site infection, or hemoperitoneum for 10 years. However, a recente peritoneal equilibration test showed a high average peritoneal transport, and subsequently decrease in ultrafiltration (UF) and difficulty in fluid balance control. After an episode of severe hypertension, patient was started at overnight stay with icodextrin (1.5 L Physioneal® 1.36%; 1.36%, 1.36%, Icodextrin).
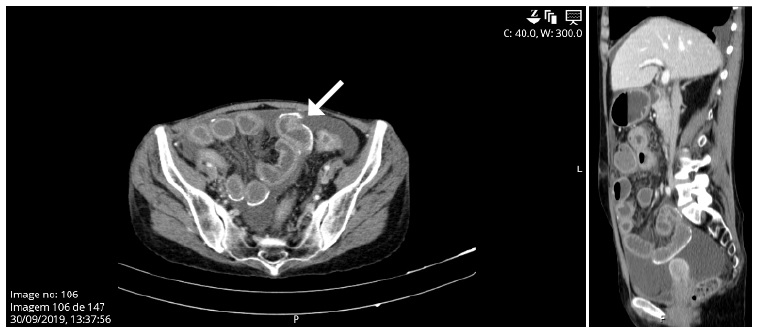
Patient has been admitted to the emergency department in distress with abdominal tenderness, nausea, vomiting, fever, and a cloudy peritoneal effluent (PE). Laboratory studies revealed hemoglobina 12.5 g/dL, C-reactive protein 1 mg/dL and an exudative PE with 5900 leukocytes/μL with 100% polymorphonuclear cells. A bacterial peritonitis diagnosis was assumed, and intraperitoneal vancomycin and ceftazidime were prescribed. Because of rapid deterioration, she was admitted to the Intensive Care Unit. After hemodynamic stabilization, she was transferred to the Nephrology department 48 hours after. The patient was diagnosed with methicillin-sensible Staphylococcus aureus (MSSA) peritonitis. The peritoneal catheter was removed 3 days after admission and the patient switched renal replacement therapy to hemodialysis (HD). Contrastenhanced abdominal computed tomography (CT) scan revealed parietal calcifications, with bowel thickening, but without bowel dilatation or signs of intraperitoneal exudates (Fig. 1).
After multiple broad-spectrum antibiotics, an undetermined febrile syndrome persisted. Fifteen days later a CT scan revealed massive ascites with perihepatic locution and peritoneal calcifications. The patient underwent percutaneous drainage. Subsequent peritoneal fluid microbiological assays were sterile. A subsequent abdominal CT scan showed a persistent perihepatic loculation, fluid in the Douglas fold, and a large epiploon. The patient repeated percutaneous drainage.
Due to an exuberant systemic inflammatory response with persistente fever and elevated inflammatory markers, severe protein-calorie malnutrition leading to total parenteral nutrition, and imaging findings, a diagnosis of EPS was assumed and treatment with tamoxifen (10 mg, twice a day) was started.
After 47 days of hospitalization, exploratory laparoscopy was then decided. Intraoperative laparoscopic findings showed exuberant peritoneal adhesions, fibrin in all quadrants, frozen loops, and multiple abdominal collections with citrine fluid but without pus. The patient was submitted to extensive enterolysis and partial removal of fibrin plaques (Fig. 2).
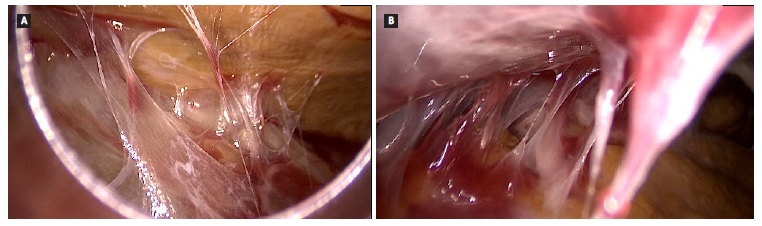
Figure 2 Findings during enterolysis. Multiple intra-abdominal adhesions of bowel loops by cobweb-like sclerotic membranes.
A clinical improvement led to a discharge after 60 days of hospitalization. Nearly three years later, the patient remains on HD. Currently, she received tamoxifen for more than 30 months with no significant abdominal complaints or adverse events and has a recently abdominal magnetic resonance imaging (MRI) with no evidence of disease progression. The patient is under evaluation for a new kidney transplant.
DISCUSSION
One of the potential rare complications of long-term PD is EPS, which is associated with high mortality, about 50% usually in the first 12 months after diagnosis.11 The etiology of EPS remains unclear, however in a pathophysiological model of a 2-hit hypothesis, the risk is based on chronic inflammation and fibrosis within the peritoneum.
Several inflammatory cytokines are elevated up to several years before EPS, suggesting the presence of chronic peritoneal inflammation as a role in the pathophysiology of EPS (the first hit).3,11
The inflammation may be associated with glucose degradation products exposure in peritoneal dialysis solutions and a major inflammation stimulus, like severe infections, which are frequently the second hit.11,12
Several studies identified the time on peritoneal dialysis and the occurrence of severe recurrent peritonitis as major risk factors for EPS.1,5,13,14 In a prospective multicenter study in Japan, 48 patients on PD were diagnosed with EPS and there was a clear association between time on PD and EPS incidence.1 It appears that PD may be continued with an acceptable risk for EPS for at least 8 years and should be carefully considered thereafter.1 There are some case reports of patients who received PD for long periods without any complications, making it difficult to decide the ideal time for HD transfer. Unfortunately, prospective data showing the benefit of preemptively switching long-term PD patients to HD are not available.1
Our patient wanted to remain in this renal replacement technique. In addition, the patient had metabolic and blood volume control, kept the same peritoneal membrane transport characteristics, and had no episodes of peritonitis, which led to postponing the decision to transition to HD. Patient was also maintained with biocompatible PD fluid to preserve residual renal function and minimize peritoneal damage. It is known that EPS increases significantly with time on technic, but most of long-term PD patients will not develop EPS.
Moreover, EPS can develop or worsen after stopping PD, and even after a transplant.11 Changes in membrane characteristics occurred 2 months before. Retrospectively, some prophylactic measures could have been considered at that time. A single center in Spain, with 679 patients on PD and 30 years of follow-up, define that EPS-prone patients as having two or more of the following characteristics: more than 3 years on PD; recurrent or severe peritonitis; presence of UF failure or high membrane transport; previous exposure to glucose PD fluids and past history of hemoperitoneum.15 The authors proposed a temporary or definitive PD switch with simultaneous treatment with tamoxifen (20 mg, twice a day), and steroids when inflammation on going is assumed. In 14 patients classified as EPS-prone, all received treatment with tamoxifen for a median of 12 months, with five patients also receiving prednisone, and one, azathioprine. Interestingly, during follow-up no patient developed EPS.15 The International Society for Peritoneal Dialysis determined that changes in peritoneal membrane function, peritonitis episodes and loss of UF are poor predictors of EPS, and there are no prospective studies that showed benefits of pre-emptively modality switching.11
Episodes of severe peritonitis (Staphylococcus aureus species, Pseudomonas or fungus) are a risk factor because they may promote peritoneal deterioration and/or encapsulation. However, EPS can develop even after a single peritoneal infection.5 In our case, we hypothesized that severe peritonitis may have been a major event in the EPS underlying process, that could be demonstrated by the development of UF failure and high transport status previous to this episode.
EPS may also develop in patients with autoimmune diseases, however, the cases reported are scarce.12,16 It was reported in a patient with systemic lupus erythematosus (SLE), without CKD, with recurrence ascites that was submitted to a peritoneal biopsy. Biopsy showed chronic fibrosing inflammation and the authors hypothesized that chronic serositis as a symptom of systemic lupus erythematosus was correlated with sclerosing peritonitis.17 Our patient had a long history of SLE that is known to cause immune-mediated serositis18 and could represent an additional risk factor to the development of EPS in patients in PD. Genetic predisposition is also discussed as a risk factor.12,19
Treatment of EPS is controversial since the first case was described in 1980.14 Nevertheless, it is relatively consensual that PD discontinuation and transfer to HD after EPS diagnosis. However, there are no randomized controlled trials to guide EPS management and the following procedures are more difficult to decide.14,20 Reported treatments include TPN and immunosuppression or antifibrotic therapy with steroids, cyclosporine, sirolimus, azathioprine, or tamoxifen.3,9,14,20,21
Tamoxifen is a nonsteroidal anti-estrogen agent used to treat breast carcinoma and fibrosing diseases.21 In animal models was demonstrated that tamoxifen blocks mesothelial-to-mesenchymal transition caused by transforming growth factor β, with consequently reduction angiogenesis and peritoneal thickness and better peritoneal function.22 The benefits of tamoxifen in EPS are based on a limited number of case reports and small series of patients and the exact mechanism is not clearly described.9,14,23 The duration and dosage of tamoxifen are not yet determined.1 In a Dutch multicenter EPS study, 24 patients were treated with tamoxifen versus 39 that were in a placebo arm. Tamoxifen has been associated with lower mortality and showed a trend of increased multivariate-adjusted survival, supporting its use to treat patients with severe EPS.23
The role of corticosteroids is still under discussion because some reports show that they are beneficial in the acute phase.1,24 In contrast, EPS can develop in renal transplant patients under steroids.11,25 A large study enrolled 111 patients, that compared patients treated with corticosteroids, tamoxifen, other immunosuppressive agents, or a combination of different treatments. The authors did not find any difference in survival between patients, despite steroids as a single agent being used only in 4 patients.7 In our case, the patient was still on a low dose of corticosteroids due to SLE, which was against the additional benefit of increasing the dose or even pulse of EV steroids.
Due to its controversial benefits and additionally an underlying HD access infection we did not associate steroids with tamoxifen treatment.
Like in our case, catheter removal is generally an option, however, in studies performed in Japan they decided to maintain the catheter to perform regular peritoneal lavage.26,27Removing mediators of the inflammatory and fibrotic process could be beneficial, and the interruption of the washout could be a trigger to the EPS worsening.10,28
Surgical intervention is a potential strategy for definitive management of EPS, despite being a complex procedure and not without risk, and the treatment of choice when irreversible intestinal obstruction occurs.10 Nonetheless, enterolysis should be considered in the early stage of the disease.10 In a retrospective observational study from Japan, 243 patients underwent surgical intervention and a total of 318 EPS surgery were performed. The patients who underwent surgery showed good outcomes (survival rates at 1, 2, 3, 5, and 8 years after EPS diagnosis were 91%, 83%, 77%, 66%, and 53% respectively). Patients in which the EPS diagnosis is being considered, should be discussed early with teams that have EPS surgery expertise.10
This case describes a successful management of EPS despite a very severe presentation, which trigger could have been MSSA peritonitis. We consider that enterolysis and tamoxifen contributed decisively to patients’ favorable response. The role of tamoxifen in prophylaxis and its association with enterolysis needs further investigation.