INTRODUCTION
Renal cortical necrosis (RCN) was first reported in 1883 by Friedlander.1 It is a rare cause of acute kidney injury (AKI) in developed countries, with a frequency of 1.9%-2% of all patients with AKI.2 It is usually bilateral and irreversible and presents with a rapid loss of glomerular filtration rate, often with oliguria, granular casts, and low-level proteinuria.3 Causes in adult population include obstetric complications, more prevalent in developing countries.2 Of the non-obstetric causes in the adult population, the most frequent etiology is septic shock.4
Drug-induced cortical necrosis is very rare (0.9% of acute cortical necrosis) and non-steroidal anti-inflammatory drugs (NSAID’s) induced cortical necrosis is even rarer.5 To the best of our knowledge, only 4 cases of renal cortical necrosis associated with NSAIDs have been described in the literature. Two of them reported acute cortical necrosis with irreversible renal failure related to ibuprofen.6 The third is a documented case of naproxen-induced renal cortical necrosis with a transiente need for hemodialysis.5 The last one is a case of anuric renal failure requiring transient dialysis in a patient receiving zomepirac, which has evolved with partial recovery of renal function.7 The classic presentation of renal cortical necrosis is an abrupt onset of oliguria or anuria, often with gross hematuria and flank pain. Urinalysis may detect hematuria, granular casts, and low-level proteinuria.2-4 RCN is characterized by confluent necrosis of the entire cortex, apart from a thin rim of viable tissue in the subcapsular, juxtaglomerular areas, and medulla. Although the pathogenesis of the disease remains unclear, the final common pathway is permanent occlusion of afferent arterioles and interlobular arteries in the cortical vasculature, either by prolonged vasospasm with secondary thrombosis, primary vascular damage with thrombosis or some combination of both.1 If this vasospasm is brief and vascular flow is reestablished, the result is acute tubular necrosis.
More prolonged vasospasm can cause necrosis and thrombosis of the distal arterioles and glomeruli, and renal cortical necrosis ensues. Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely prescribed in general clinical practice and are readily available over the counter. NSAIDs can induce several different forms of kidney injury including hemodynamically mediated AKI, electrolyte and acid-base disorders, acute interstitial nephritis, nephrotic syndrome and papillary necrosis, most of which are self-limiting when the drug is stopped.8 The risk of renal toxicity is potentially increased in situations where there is stimulation of the renin-angiotensin system, such as with volume depletion, or in pre-existing chronic kidney disease. NSAIDs may cause AKI by blunting this counter-regulatory renal response of prostaglandins release that counteract vasoconstrictors to maintain renal blood flow.
Severe renal hypoperfusion could conceivably lead to acute cortical necrosis and irreversible acute renal failure.6,9
CASE REPORT
We present the case of a 20-year-old black man with no significant past medical history, who was admitted to the hospital due to bilateral flank pain and decreased urine output. He had been symptomatic for two weeks with severe toothache due to a dental abscess. Since the pandemic outbreak of COVID-19 he was unable to contact any dentist and he was exceeding the maximum recommended doses of paracetamol and ibuprofen. At hospital admission, he had normal blood pressure and a slight bilateral pedal edema. Initial laboratory tests revealed anemia (Hb 11 g/dL), a slight increase in inflammatory parameters and acute renal failure (sCreatinine 12.7 mg/dL, sUrea 109 mg/dL) with a bland urinalysis and a urine protein-to-creatinine ratio of 1.8 g/g. Renal ultrasound excluded obstruction and a Doppler ultrasonography was obtained with no signs of renal blood vessel occlusion. Viral serologies were negative, clonal gammopathies were excluded, autoimmune study and serum complement levels were normal. Blood and urinary cultures were also negative (Table 1). A non-contrast abdominal and pelvic computed tomography (CT) scan was performed with no positive findings. He underwent tooth extraction in the hospital and completed 10 days of amoxicillin-clavulanate and metronidazole with resolution of infection. Despite proper fluid replacement, the patient showed no clinical improvement and presented with anuria, so hemodialysis was started. Since he was anemic after dental extraction, a renal biopsy was not immediately performed, and the patient was discharged and referred to a hemodialysis program.
Table 1 Laboratory results
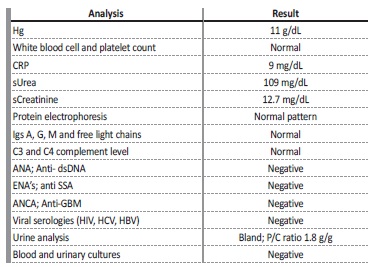
Hg - hemoglobin; CPR - C reactive protein; s - serum; Igs - immunoglobulins; ANA - antinuclear antibody; Anti-dsDNA - anti-double stranded DNA; ENAs - extractable nuclear antigen antibodies; anti SSA - anti Sjögren’s syndrome related antigen A autoantibodies; ANCA - anti-neutrophil cytoplasmic antibody; Anti-GBM - anti-glomerular basement membrane antibody; HIV - human immunodeficiency virus; HCV - hepatitis C virus; HBV - hepatitis B virus; P/C ratio - urinary protein to creatinine ratio
Given the absence of renal recovery, the patient was readmitted for renal biopsy 6 weeks after the previous hospital admission. It showed 27 glomeruli, all of them obsolete, with multiple cortical foci of ischemic necrosis that did not extend into the medulla, suggesting extensive cortical necrosis. There was 75% of interstitial fibrosis and no arterial thrombi were detected (Fig. 1). At that moment, we were able to conclude that the diagnosis was renal cortical necrosis, probably secondary to NSAIDs intoxication. The patient became dependente on renal replacement therapy.
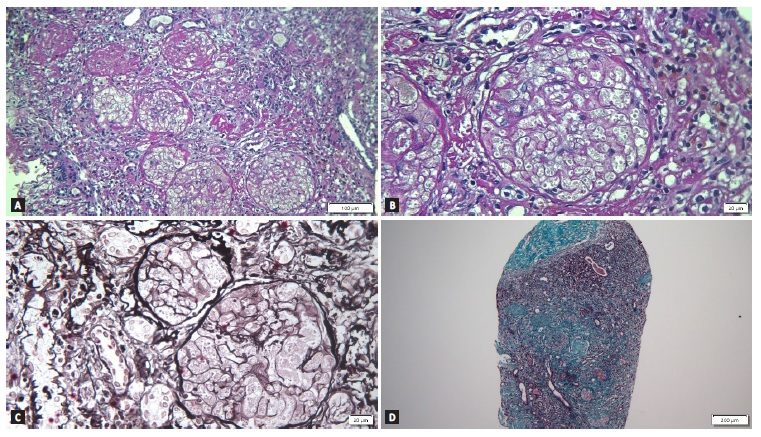
Figure 1 A - Periodic acid-Schiff (PAS) stain showing obsolete glomeruli with multiple cortical foci of ischemic necrosis, suggesting extensive cortical necrosis on light microscopy (magnification x 160); B - Previous image magnified showing absence of cell nuclei in the glomeruli (magnification x 400); C - Periodic acid-methenamine silver stain (magnification x 400); D - Masson’s trichrome stain showing severe interstitial fibrosis (magnification x 60).
DISCUSSION
The COVID-19 pandemic has had a major impact on the capacity of health systems to continue delivering essential health services. We present this case to illustrate the impact of delaying non-urgent dental care which can lead to irreversible kidney damage.
In this case, the clinical presentation of flank pain and anúria required the exclusion of a vascular etiology, besides urinary tract obstruction that was ruled out by the renal ultrasound. For this purpose, a contrast-enhanced CT should have been performed. However, at that time the decision made was to not use contrast in the contexto of acute kidney injury. Despite its low sensitivity, a Doppler ultrasonography was performed with no signs of renal blood vessel occlusion.
In addition, the patient had no pro-thrombotic risk factors such as nephrotic syndrome, history of neoplasm, coagulopathy, or a major trauma, and the occurrence of bilateral thrombosis seemed unlikely.
A non-contrast abdominal and pelvic CT scan was performed since the patient presented with abdominal/flank pain of uncertain etiology. It allowed us to exclude other diagnoses, such as renal colic, pyelonephritis, or intra-abdominal complications, even though they seemed unlikely in the absence of fever, sustained elevation of inflammatory parameters and the fact that the patient was never septic. If a contrast enhanced CT scan had been performed, maybe signs of cortical necrosis could have been seen, such as the non-enhancement of the renal cortex against a background of intact medullary perfusion and lack of excretion of contrast to the collecting system. These features are pathognomonic for acute renal cortical necrosis and diagnosis could have been made earlier, without the need for an invasive procedure.10 However, renal biopsy is the gold standard for diagnosis and adds prognostic value.1 In our case, we found cortical necrosis with sparing of the medulla and 75% of interstitial fibrosis, which we did not expect. Renal biopsy was performed 8 weeks after the onset of NSAIDs initiation, which may justify this finding and suggests the contribution of NSAIDs in the etiology of our patient’s renal failure. It should be noted that this is a case of cortical necrosis that presented with a bland urinalysis. Although this finding is not the most characteristic of cortical necrosis, it should not exclude this differential diagnosis. Drugs, especially non-steroidal anti-inflammatory drugs (NSAID’s), are very rarely described in the literature to cause cortical necrosis.
A direct cause-and-effect relationship between NSAIDs and acute cortical necrosis cannot be established unequivocally. In our case, the absence of other causes for cortical necrosis, the temporal relation between NSAIDs intake in high doses and the onset of clinical presentation of renal failure, as well as the recognized previous published cases of acute cortical necrosis related to NSAIDs, made this association unavoidable. The use of NSAIDs in the general population is safe and efficacious when used in therapeutic dosages for a limited period of time. Despite being readily available, a subset of individuals are susceptible to serious renal toxicity and caution should be exercised when these drugs are used.















