INTRODUCTION
Point-of-care ultrasound (POCUS) is an emerging bedside discipline that aims to enhance physical examination findings with real-time information from the ultrasound. As opposed to a comprehensive examination performed by a radiologist, POCUS is intended to answer focused clinical questions and is performed by the same physician examining the patient1.
Peritoneal dialysis (PD) catheter-related infections (PD-CRI) are a frequente complication of this renal replacement therapy technique and a major risk factor for PD-related peritonitis2. The diagnosis of PD-CRI is made with physical examination (PE), but clinical judgment is often required to decide whether to initiate therapy or to follow carefully. The aim of this study is to evaluate the diagnostic accuracy of POCUS in PD-CRI.
MATERIALS AND METHODS
This is a single-center prospective observational study. All of our PD catheters are double-cuffed, with swan-neck conformation, and placed under laparoscopy, with careful rectus sheath and intracutanous suturation.
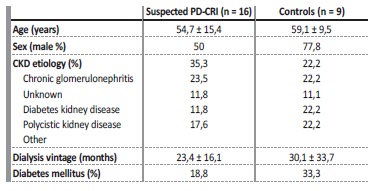
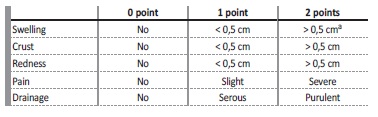
The study protocol was approved by the ethical committee of our hospital and written consent was obtained from participating patients. Sixteen PD patients with PD-CRI clinical suspicion were enrolled in this study between April and June of 2018. We also recruited nine patients who were free from clinically diagnosed PD-CRI in the last three months and antibiotic therapy in the last month, as controls. PD-CRI was defined as a purulent discharge with or without inflammatory signs in the PD catheter exit-site or subcutaneous tunnel, and a positive microbiological culture collected from the exit-site. PE findings were coded by our PD nurses using the Exit-site Scoring System (ESS) modified from Schaefer F et al3,4 (Table 1).
Table 1 Exit-site Scoring System adapted from Schaefer et al.(3). A score ≥ 4 or ≥ 2 with purulent drainage is suggestive of PD-CRI. A - or tunnel involvement
POCUS was performed by a nephrology fellow with previous ultrasound experience using a MySono U6 ultrasound machine (Samsung Medison, Seoul, South Korea) with a linear probe (5-12 MHz), after applying a transparent adhesive film-dressing at the exit-site to ensure no contamination. POCUS examination was performed on the same day as the PD catheter clinical evaluation and was repeated at every patient visit. We considered a positive POCUS as any anechoic collection in relation to the external cuff, catheter tunnel or internal cuff, and its largest dimension was measured in millimeters, either in transverse or longitudinal view, whenever present.
As the aim of this observational study was to evaluate the diagnostic accuracy of POCUS in PD-CRI, no clinical decisions were made by the PD specialists based on the ultrasound findings.
STATISTICAL ANALYSIS
Statistical analysis was performed using IBM SPSS, version 25.0 for Windows software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median (range) if normally or non-normally distributed, respectively. Categorical variables were represented by absolute and relative frequencies.
Comparisons between groups were performed using Chi-squared test for categorical variables and Student’s t-test or Mann-Whitney U test for continuous variables if normally or nonnormally distributed, respectively. Diagnostic accuracy of PE and POCUS was assessed with the area under the receiver operating characteristic curve (AUROC) and was reported with its corresponding 95% confidence interval (CI). A p-value of less than 0.05 was considered statistically significant.
RESULTS
Between April and June 2018, a total of 13 PD-CRIs were diagnosed, from 22 suspected cases in 16 patients. Fifty percent of these patients were male and their average age was 54,7 ± 15,4. Regarding the nine patients in the control group, 77,8% were male and their average age was 59,1 ± 9,5. The baseline characteristics of the study population can be found in Table 2.
The mean ESS score for the suspected cases of PD-CRI was 2,82 ± 1,22, and the most commonly encountered sign was purulent discharge (90,9%). In PD-CRI cases the mean ESS score was 3,08 ± 1,44 and all cases presented with purulent discharge. The median antibiotherapy duration for PD-CRI was 22,5 days [IQR 14-28] and the most commonly isolated agent was Corynebacterium spp (42,8%). The most frequently used antibiotics were ciprofloxacin, flucloxacillin and cotrimoxazole.
Furthermore, suspected PD-CRI cases with purulent drainage and a negative microbiological culture, had similar PD-CRI rates as the control group, during a six-month follow-up (p = 0,782).
POCUS was performed at every patient visit, for a total of 69 evaluations during follow-up. All PD-CRI cases had a positive POCUS, with distinct anechoic collections around the external cuff or the catheter in the subcutaneous tract (Figure 1). In contrast, none of the controls had a positive POCUS (p < 0,001). Also, PD-CRI patients had significantly thicker collections than non-confirmed cases (p = 0,001).
The AUROC of PE for the diagnosis of PD-CRI was 0,6 (95% CI 0,37-0,84) (Figure 2A). Purulent drainage showed a sensibility of 100% and a specificity of 61,9% for the diagnosis of PD-CRI. In contrast, POCUS had na AUROC of 0,91 (95% CI: 0,73-1) for the diagnosis of PD-CRI
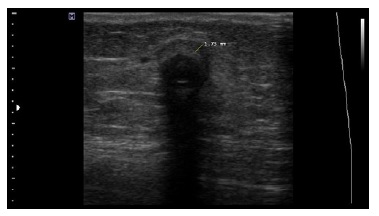
Figure 1 Anechoic fluid collection around the PD catheter external cuff measuring 1.7 mm in a patient with PD-CRI
(Figure 2B). The optimal cut-off point of the collection dimension in our population was ≥ 1,4 mm (Sensitivity 100%; Specificity 89%). In 14 initial POCUS evaluations (four patients had more than one evaluation for clinical suspicion of PD-CRI), the collection dimensions were ≥ 1,4 mm. In all, but one case, the diagnosis of PD-CRI was considered.
A collection thickness at the time of diagnosis ≥ 1,4mm was associated with PD-CRI relapse in less than a month (p = 0,04), and new episodes of PD-CRI or peritonitis in a 6-month follow-up (p = 0,001).
A total of three patients had their PD catheters removed and all of them had significant residual collections in the intercuff or internal cuff after treatment (≥ 1.4 mm).
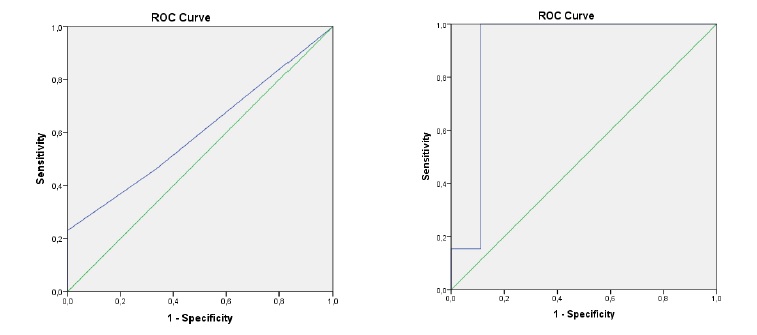
Figure 2 A - Receiver operating characteristic (ROC) curve for physical examination, using the ESS score. Area under the ROC curve (AUROC) for physical examination is 0,6 (95% CI 0,37-0,84). B - Receiver operating characteristic (ROC) curve of POCUS. Area under the ROC curve (AUROC) for POCUS is 0,91 (95% CI: 0,73-1)
Antibiotic cessation was determined clinically, at which point we verified a significant reduction of the collection´s thickness (mean dimensions at the time of diagnosis 3,6 ± 2 mm; mean dimensions at the end of antibiotic treatment 1,9 ± 1,2 mm; p = 0,028), which was progressive during treatment. Suspected PD-CRI cases with negative POCUS and not exposed to antibiotic therapy, had similar PD-CRI rates as the control group, in a one-month and six-month follow-up (p = 0,929 and p = 0,302, respectively).
DISCUSSION
In clinical practice, PD-CRI is diagnosed by the presence of purulent drainage, with or without inflammatory signs of the skin or subcutaneous tunnel. Unfortunately, these clinical signs are not specific and they can also represent a local skin reaction or trauma4. In addition, these signs are not always present. In a series of 126 exit-site infections, 45 did not have a purulent discharge and 32 exit-sites had a normal appearance5. Previous studies also have shown that ultrasound can detect clinically occult tunnel infections6,7.
In this study, the mean ESS score of our PD-CRI patients was 3,08, which represents a clinical picture with a low burden of signs and symptoms. This may explain why the PE of the exit-site had low accuracy for the diagnosis of PD-CRI. In our series, purulent drainage was present in every case of PD-CRI (100% sensitivity), but it showed a very low specificity (61,9%) for the diagnosis. Relying on this sign can increase unnecessary exposure to antibiotics, as exit-site microbiological testing can take days to have a result. In fact, culture-negative suspected PD-CRI cases with purulent discharge did not experience an increased number of PD-CRI or peritonitis compared to controls, at six months. A possible explanation is that some cases of purulent discharge may represent a self-limited ESI, which might be safely handled with close surveillance and without antibiotic exposure.
Another possible explanation is that, since all our patients apply a mupirocin dressing as part of exit-site care, some of the drainage considered purulent by our nurses is, in fact, not truly purulent.
Although there are broad confidence intervals for the AUROC of both PE and POCUS, probably related to the low number of patients enrolled in this study, POCUS showed high accuracy for the diagnosis of PD-CRI. All of the cases had a positive POCUS evaluation (100% sensitivity), and collections ≥ 1.4 mm were highly specific for the diagnosis (89%). Also, none of our controls had a positive POCUS evaluation. This was similarly reported in a previous ultrasound study by Kwan et al8. Finally, suspected PD-CRI cases with negative POCUS had similar PD-CRI rates as the control group, at a one-month follow-up, which is reassuring for the clinician.
In our center, PD-CRI are treated as per ISPD guidelines4 - at least two weeks of effective antibiotics or at least three weeks in tunnel envolvement or caused by Pseudomonas spp, as reflected in the results.
External-cuff shaving is usually considered when there is no significant improvement after four weeks of antibiotics or after an early relapse. In difficult to treat Corynebacterium spp PD-CRI, local instilations with gentamicin or intra-peritoneal vancomycin are considered. While the aim of this study was only to assess the diagnostic accuracy of POCUS, and the information of the POCUS evaluation was not used by PD specialists for clinical decisions, there is prognostic information worth discussing. We found that the size of the collection at the time of diagnosis is associated with adverse outcomes, such as relapses or peritonitis. This is consistent with previous findings in the literature9,10. Although we believe that nephrologists will not perform invasive treatments such as cuff shaving or PD catheter removal without a trial of antibiotics, besides improving diagnosis, POCUS could also hint to the clinician which patients will likely fail conservative treatment. Reflecting on our results, we had a high incidence of new PD-CRI or peritonitis compared to controls, which should probably prompt a more proactive approach, such as cuff shaving, when significant collections remain after antibiotic treatment.
Likewise, we documented a significant decrease in the collection dimensions during treatment. This could be used to monitor therapy response, as suggested by previous studies, but requires an initial evaluation before treatment initiation8,9. The study of Vychytil et al, suggests an ultrasound control examination at 2 weeks of antibiotic therapy in S. aureus PD-CRI. A 30% reduction of the pericatheter hypo-echoic zone indicates response to therapy and reduces the risk of catheter removal9.
The persistence of a hypo-anechoic area around the cuff or the catheter, of more than 1 mm after a 2 week course of antibiotic therapy or involvement of the internal cuff, is usually a sign of poor catheter prognosis and elevated risk of peritonitis8,9. In the situation of infection with internal cuff involvement catheter removal is usually recommended11.
This study has obvious limitations, such as its single-center nature and the small number of patients. In addition, POCUS evaluation was performed by only one operator which could introduce a significant bias in the collection measurements. Although, POCUS information was not used for clinical decision-making, the PD specialists were not blinded while POCUS was being conducted, which could also induce unconscious bias.
CONCLUSION
Although there are plenty of ultrasound studies focusing on PD-CRI, in all of these research projects the ultrasound evaluation was performed by a radiologist. In clinical practice, radiology-performed ultrasound means the use of more resources, more patient visits and are a lot more timeconsuming.
We believe this is one of the reasons why ultrasound is not used more often in this situation. Our study shows that nephrologist-performed POCUS, using ultrasound as an extension of the physical examination at the bed-side by the attending clinician, is not only feasible, but also superior to PE for the diagnosis of PD-CRI, when there is a clinical suspicion of infection. POCUS findings are also of prognostic importance.
POCUS in PD-CRI is simple, quick, easy to perform, and safe to the patient, and should be considered by nephrologists with access to na ultrasound machine.