INTRODUCTION
Incremental peritoneal dialysis (PD) was first described in the late 1990s. Since then, the definition used in studies has varied wildly1. Recently, incremental dialysis has been established as a strategy, with a clear intention to increase the dose of PD, as a consequence of renal clearance declines or onset of uremic symptoms. The initial prescription should be lower than standard “full‑dose” PD, and the initial peritoneal clearance should be lower than the individualized clearance goal2.
In 2006, the International Society for Peritoneal Dialysis (ISPD) published a guideline, primarily focusing on targets for small solute removal and ultrafiltration. However, the relationship between small solute clearance and clinical outcomes has always been particularly weak, without any impact on dialysis‑related morbidity and mortality3.
Also, health‑related quality of life (HRQoL) remains poor concerning the overall population3. For this, since 2014, transition of care in chronic kidney disease has received focused attention, with the optimization of other dimensions of dialysis delivery accepted nowadays. These include quality of life, psychological burden, inflammation and malnutrition, which can improve patient outcomes with a paradigm shift from a one‑size‑fits‑all approach to a goal‑directed dialysis4.
The concept of incremental PD is inherent to new ISPD recommendations, allowing for an individualized and person‑centered prescription, providing better quality of life, fewer exchanges and a smaller solution storage space, something which is often cited as a drawback of PD5,6.
Although some authors have claimed potential clinical advantages using an incremental approach, the studies are few and heterogeneous7,8,9,10.
Our purpose is to show the possibility of incremental approach without risks for patients. As such, we followed a retrospective design, focused on the comparison between incremental and full‑dose PD strategies.
The authors believe that the results of this study may reinforce the adoption of an incremental approach for patients who initiate PD.
METHODS
General Design and Definitions
Following an observational and retrospective design, we assessed demographic, clinical and analytical data from patients who started PD to address end‑stage renal disease (ESRD), in our center, between January 1999 and December 2018.
Our main objective was to disclose the chance of an incremental approach when patients began peritoneal dialysis. For this purpose, we used the definition of Incremental PD according to the 1997 NKF‑DOQI clinical practice guidelines for peritoneal dialysis adequacy11. So, we consider the incremental approach the start of PD on continuous ambulatory peritoneal dialysis (CAPD) using three 1.5‑2L dwells daily, seven days a week.
Conversely, we defined full‑dose PD prescription as CAPD with 4 or more dwells daily. We excluded patients on automated peritoneal dialysis (APD) because the incremental prescription is not established in this strategy2.
The study complied with the principles of the Declaration of Helsinki and the ethical requirements of our center for retrospective observational studies.
Study Population
For this study, we recruited all patients starting PD in our unit for ESRD treatment, between the 1st January of 1999 and the 31st December of 2018. The follow‑up was closed on the 31st December of 2019. We excluded from the analysis patients < 18 years of age, PD urgent start, patients with a follow up on PD < 1 year, patients on APD and patients who were not PD first. Finally, we also excluded patients with unsuitable clinical records.
PD patients were assigned preferably to an incremental prescription when they had a significant residual renal function (RRF) and a full dose of peritoneal clearance was not immediately required. PD prescription was adjusted to a full dose PD regimen following renal clearance declined or onset uremic symptoms.
„ Study Variables and strategy of analysis
We reviewed manual medical charts and the hospital’s electronic database to collect demographic, clinical and analytical data. Clearances were measured as recommended by international guidelines12,13. Overhydration was obtained by multifrequency impedance with BCM® Monitor.
For our study, we explored:
1) Patients’ features related to incremental prescription;
2) Differences between patients on incremental and full dose PD, according to clinically and analytical adequacy;
3) Outcomes of both approaches: RRF, peritonitis incidence, patient survival and technique survival.
Statistical analysis
Univariate comparisons between groups were performed resorting to the Pearson chi‑square test and the t‑test or Mann‑Whitney U test, under normality assumption, for categorical data and continuous variables, respectively. We used non‑parametric paired tests to compare rates of RRF decline. To assess the risk of incremental prescription in the occurrence of peritonitis, we used a multiple binary logistic regression complemented with a multinomial model. Time to event analysis for incremental longevity and technique or patient survival was performed resorting to Kaplan‑Maier estimates and a multivariate Cox proportional hazards model. An alternative competing risks model was obtained to give robustness to our results. We used the R project® software for statistical analysis, with an assumed significance level of 5%.
RESULTS
Population Overview
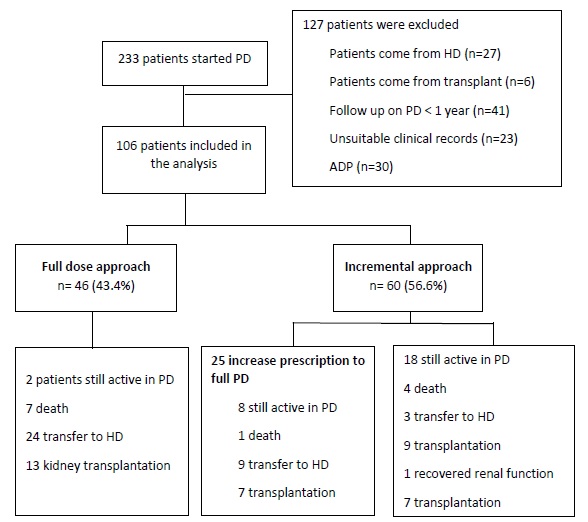
During the study period, 233 patients started PD in our center. From those, 127 patients did not fulfil the study criteria, so we included 106 patients in our analysis (Figure 1).
In our population, there was a minor predominance of the male gender, 56 (53%), with a mean age of 49.1 ± 14.2 (range 21 - 79) years. A quarter of the patients were diabetic (8% type 1 and 16% type 2). The leading causes of ESRD were chronic glomerulonephritis, 34 (32%) and diabetic nephropathy, 20 (19%). At the end of the study, 36 patients (34%) were transferred to hemodialysis and 29 patients (27%) were transplanted. As a whole, there were 12 deaths (11%), one patient recovered renal function and 28 patients (27%) were still active in PD. The mean follow‑up was approximately 4 years (45.1 ± 24.6 12 - 105 months).
„ Incremental prescription
The series included 60 (57%) patients under an incremental approach. Table 1compares the demographic and clinical characteristics of incremental PD and full dose PD. More patients in incremental PD were female (62% vs 28%, p < 0.001*) and had a better residual renal function, represented by higher urinary output (1225mL IQR(900;1620) vs 700mL IQR(550;1200), p = 0.001*) and glomerular filtration rate (6.3 mL/min/1,73m2 IQR(4,4;8,7) vs 3.6 mL/min/1.73m2 (2.6;5.6), p < 0.001*). The decade of PD start was also significant, as 82% of the patients who started PD in the last decade began with na incremental approach, against only 18% who had begun in the previous decade (p < 0.001*). There were no significant differences between age, etiology of kidney disease, presence of diabetes or anthropomorphic characteristics.
PD adequacy was evaluated regularly according to international guidelines. Table 2 displays some clinical parameters of adequacy, in the first evaluation after PD start and after a 12‑month follow‑up.
As expected, after one year, the Incremental group still had better RRF and higher solute removal (Kt/V 2.37 ± 0.59 vs 2.14 ± 0.66, p = 0.033*) which certainly contributed for better phosphatemia (4.6 ± 0.7 mg/dL vs 5.2 ± 1.2 mg/dL, p = 0.016*) and lower overhydration (0.6 ± 1.3 L vs 1.3 ± 1.8 L, p = 0.196).
Table 1 Demographic and clinical differences according to study group
| Full dose PD | Incremental PD | p value | |
| Age (years) | 48.7 ± 15.2 | 49.4 ± 13.6 | 0.807 |
| Gender (females) (%) | 13 (28) | 37 (62) | 0.001* |
| Kidney disease (%) Glomerular Interstitial Hypertension Diabetic nephropathy Other/Unknown | 17 (37) 2 (4) 5 (11) 9 (20) 13 (28) | 17 (28) 5 (8) 10 (17) 11 (19) 17 (28) | 0.760 |
| Diabetes (%) | 10 (22) | 15 (25) | 0.872 |
| BSA (m2) | 1.76 ± 0,18 | 1.70 ± 0.18 | 0.108 |
| Body mass index (Kg/m2) | 24.8 ± 3.3 | 24.7 ± 3.7 | 0.898 |
| GFR1 (per mL/min/1.73m2) | 3.6 (2.6 ; 5.6) | 6.3 (4.4 ; 8.9) | < 0.001* |
| Urinary output1 (mL) | 700 (550 ; 1200) | 1225 (900 ; 1625) | 0.001* |
| PD start (%) 1999-2008 2009-2018 | 31 (67%) 15 (33%) | 11 (18%) 49 (82%) | < 0.001* |
1 Excluding anuric patients. Figures denote mean values ± standard deviation, median with interquartile range (numerical variables) or absolute numbers (%) (categorical variables). Comparison by χ2 distribution, t‑test and one‑way ANOVA. PD - Peritoneal dialysis; BSA - Body Surface Area; GFR - Glomerular Filtration Rate
Table 2. Clinical parameters of adequacy at PD start and after 12 months
| Full dose PD | Incremental PD | p value | |
| Hemoglobin (g/dL) At the beginning After 12 months | 11.2 ± 2.1 10.9 ± 2.1 | 11.5 ± 1.8 11.4 ± 1.7 | 0.612 0.309 |
| Albumin (g/dL) At the beginning After 12 months | 3.7 ± 0.5 3.5 ± 0.6 | 3.5 ± 0.4 3.6 ± 0.4 | 0.066 0.308 |
| Phosphate (mg/dL) At the beginning After 12 months | 4.8 ± 1.1 5.2 ± 1.2 | 4.4 ± 1.1 4.6 ± 0.7 | 0.096 0.018* |
| Overhydration (L) At the beginning After 12 months | 2.2 ± 2.5 1.3 ± 1.8 | 1.5 ± 1.9 0.6 ± 1.3 | 0.359 0.196 |
| GFR (mL/min/1.73m2) At the beginning After 12 months | 4.5 ± 2.9 3.5 ± 3.2 | 6.5 ± 3.2 5.7 ± 3.2 | 0.001* 0.001* |
| Total weekly Kt/V At the beginning After 12 months | 2.30 ± 0.59 2.14 ± 0.66 | 2.44 ± 0.58 2.37 ± 0.59 | 0.204 0.033* |
| Urinary Output (mL) At the beginning After 12 months | 700 (550 - 1200) 600 (205 - 1215) | 1225 (900 - 1625) 1000 (740 - 1425) | 0.001* 0.001* |
Figures denote mean values ± standard deviation. Comparison t‑test or Mann‑Whitney U test, according to normality assumption. PD - Peritoneal dialysis; GFR - Glomerular Filtration Rate
During the follow‑up period, to achieve clearance and therapy goals, 25 patients (42%) had a peritoneal prescription increase to full‑dose, although only three patients (5%) during the first year. At the end of the study, 18 patients (30%) were still in incremental PD, while 17 patients (28%) finished PD therapy during the incremental approach: 9 patients received a kidney transplant, 4 patients died, 3 patients dropped out to hemodialysis and one patient recovered renal function. Censoring for PD drop out to hemodialysis, death, kidney transplant and renal function recovery, the median time of Incremental PD prescription was 53.2 months (IC 95% 34.4 - 92.6) and one, two and three‑year longevity probabilities were 95%, 85% and 56%, respectively.
Residual Renal Function Decline
During the first year, twelve individuals became anuric, three of them from the Incremental group. When we analyzed diuresis as a surrogate marker for the RRF, we could observe a significant decline in both groups. The mean decline during the first year was 208mL (p=0.004*) in the incremental group, and 237mL (p=0.008*) in the full‑dose group. Comparing GFR also showed similar significant losses in both groups, as the mean reduction for the incremental group was 1.2 mL/min (p = 0.001*) and for the full‑dose group was 0.7 mL/min (p = 0.024*).
Peritonitis
We recorded 108 peritonitis in 60 patients (1 episode per 44 months). When we applied a multivariate binary logistic regression with variables such as age, gender, BMI, diabetes or incremental PD, none had a significant protective effect for the occurrence of at least one peritonitis. However, a multivariate multinomial model (with the same variables) showed that the protective effect of Incremental PD is progressive, as revealed by a significant decrease in the probability of 2 or more peritonitis (OR 0.35 0.14 - 0.87, p = 0.046*), whereas only a non‑significant reduction occurred for one peritonitis (OR 0.63 0.24 - 1.68, p = 0.398), when compared to peritonitis‑free patients (Figure 2).
„ Technique and Patient Survival
Until the end of the study and considering hard outcomes, 36 patients (34%) were transferred to hemodialysis, whereas 12 patients (11%) died. For the purpose of this study, no significant diferences were recorded for patient survival when analyzing the incremental approach impact. The median time estimate to hemodialysis transfer was 79 months (CI95% 61‑105).
The univariate analysis revealed a significant delay to those on incremental prescription (technique survival at 5 years: 74% vs 49%, p = 0.03*, log‑rank).
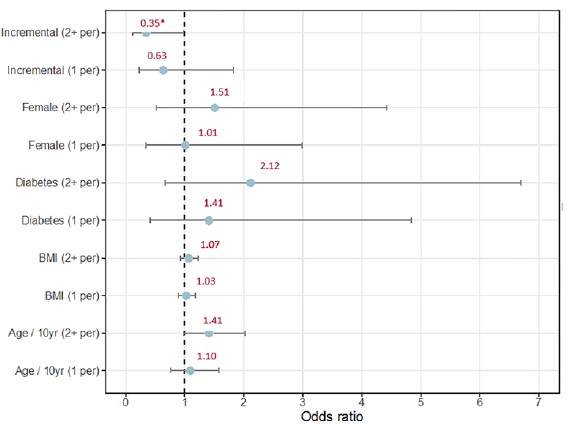
Figure 2. Predictors of peritonitis. Multivariate logistic regression analysis Multivariate multinomial regression analysis. The horizontal line represents confidence intervals and the point in the horizontal line represents the odds ratio. Incremental dialysis had a significant protective effect on the probability of 2 or more peritonitis. DM - Diabetes mellitus; BMI - body mass index; APD - Automated peritoneal dialysis; yr - years; 1 per - 1 peritonitis; 2+ per - 2 or more peritonitis
This result was reinforced by a significant protective impact of incremental approach (OR 0.41 0.19 - 0.92, p = 0.030*) on the risk of drop out to hemodialysis in a multivariate Cox proportional hazards model (Table 3), even with the use of possible variable confounders, such as the occurrence of peritonitis in the first year, RRF or the PD start decade.
Table 3. Predictors of drop out to hemodialysis. Multivariate analysis
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (per decade) | 0.97 | 0.75-1.26 | 0.814 |
| DM | 1.52 | 0.69-3.34 | 0.303 |
| Albumin (g/L) | 0.89 | 0.38-2.11 | 0.798 |
| Body mass index (per Kg/m2) | 1.0 | 0.89-1.13 | 0.999 |
| Peritonitis in the first year | 1.83 | 0.79-4.27 | 0.158 |
| PD start decade | 1.74 | 0.77-3.91 | 0.182 |
| GFR (mL/min) | 0.94 | 0.82-1.08 | 0.401 |
| Incremental approach | 0.41 | 0.19-0.92 | 0.030* |
Best model. Logistic regression analysis OR - Odds ratio; CI - Confidence interval; DM - Diabetes mellitus; PD - Peritoneal dialysis; GFR - glomerular filtration rate.
Using an alternative competing risks model, the probability of drop out to hemodialysis at 5 years was likewise better for incremental PD patients than for those on full‑dose PD (19% vs 38%, p = 0.033*). For the other events of the analysis (Table 4) there were no significant differences, as the 5‑year probability for death was 9% vs 13% (p =0.423), and for transplant was 22% vs 20% (p = 0.835).
Table 4. Technique or patient survival
| Event | Variable | Hazard p (36 months) | Hazard p (60 months) | HR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Death | Full dose prescription | 7 % | 13 % | 0.62 | 0.20 - 1.98 | 0.423 |
| Incremental | 4 % | 9 % | ||||
| Transfer to HD | Full dose prescription | 17 % | 38 % | 0.47 | 0.23 - 0.44 | 0.033* |
| Incremental | 14 % | 19 % | ||||
| Transplant | Full dose prescription | 14 % | 20 % | 1.09 | 0.52 - 1.25 | 0.835 |
| Incremental | 16 % | 22 % |
Competing Risks Models. Risk of drop‑out to hemodialysis, death and transplantation between incremental and full PD approach PD - Peritoneal dialysis; Tx - kidney transplantation; HR - Hazard ratio
DISCUSSION
According to our results, incremental peritoneal dialysis was associated with lower rates of two or more peritonitis and a significant delay in technical failure and drop out to hemodialysis.
In the past, the incremental approach was often mistaken for a way to start dialysis earlier14. The potential benefits of this modality, including improved quality of life, reduced glucose exposure, better peritonitis‑free survival and longer preservation of RRF, along with the recognition of the modern concept of individualized prescription, have widespread this strategy as a way to start PD worldwide15.
However, few observational and randomized trials confirmed these advantages as well as the positive effects on mortality and technique failure.
In our population, females, patients with higher RRF (higher GFR and UO) and patients who began PD recently, were risk factors to receive more often the object of an incremental strategy. While RRF affected the clinical decision of patient allocation (incremental approach vs full dose PD), the unintentional impact of gender can be explained by a lower urea distribution volume in women than men, achieving higher Kt/V with fewer daily exchanges16.
Phosphate plays a pivotal role in the development of secondary hyperparathyroidism and it is a significant predictor of cardiovascular mortality, together with fluid overload17,18. On PD, phosphate balance is complex, depending upon RRF and peritoneal phosphate clearance.
After one year, our study found lower levels of phosphatemia in patients under an incremental strategy in comparison with patients under full dose PD. As peritoneal phosphate clearance is slower than urea and creatinine clearance, longer exchanges such as those which occur in an incremental approach, may provide an advantage for phosphate clearance19. Despite this, we know that superiority, in phosphate control, is attributable also to better RRF in an incremental approach.
In the last 20 years, there has been a marked decline in peritoneal infections (PIs) after the introduction of Y‑set, double bag systems and prophylactic antibiotic before PD catheter insertion20,21. Nevertheless, PIs remain a significant source of morbidity, technique failure and mortality among PD patients22,23. Even though many studies have identified controversial non‑modifiable and modifiable predictors of peritonitis24,25,26, exit‑site and catheter‑tunnel infections were consistently considered major predisposing factors, favoring recommendations for early peritoneal catheter insertion and accurate management of Staphylococcus aureus carriage, with prophylactic antibiotic use to avoid PIs27,28. On the other hand, the number of exchanges seems to be also a critical determinant of peritonitis29. In this context, this study showed a significantly lower risk of two or more peritonites incidence with an incremental approach, perhaps due to less frequente connections and consequent inferior touch contamination. One study that similarly compares the two approaches did not show statistically significant differences, although patients were preferably treated with APD rather than with CAPD9. However, similarly to our results, a study that compared a regime of 3 and 4 exchanges showed a tendency towards lower susceptibility for peritonitis in patients undergoing 3‑exchange7.
Over the years, the relevance of RRF and urinary output (UO) as favorable prognostic factors on morbidity, mortality, and quality of life has grown30. Apart from solutes clearance, RRF maintains fluid balance and contributes to essential endocrine functions, including secretion of erythropoietin and synthesis of 1.25‑dihydroxycholecalciferol.
Strategies aimed to preserve RRF and UO as biocompatible solutions and renin‑angiotensin‑aldosterone system blockage are of paramount importance to dialysis patients31,32,33.
The incremental approach is associated with lower glucose exposure because patients received fewer exchanges and often one icodextrin exchange could be a strategy to preserve RRF and UO. However, our study did not show this protective effect.
Previous studies notwithstanding, at present, technique and patient survival associated with a better quality of life are the most critical challenges in peritoneal dialysis. The widespread use of PD has been limited due to some reluctance of patients and physicians to choose this dialytic modality, traditionally associated with early technique failure34. Some reports showed similar results for technique failure and patient survival among patients dialyzed with 3 or 4 exchanges35,36.
In our study, the risk of drop out to hemodialysis between patients under an incremental approach was lower than in full dose PD (p=0.033*). Only incremental strategy was associated with better outcomes in technique failure and consequent drop out to hemodialysis.
Although we did not evaluate total glucose exposure, the lower risk of drop out to hemodialysis in patients who begin PD under na incremental approach could be due to peritoneal membrane ultrastructure transformations by glucose and GPD susceptibility37,38. In our study, the occurrence of peritonitis did not show any significant relation with technique failure. These results are in concordance with previous studies, although they have not assessed the effect of 3‑PD exchange per day.
There are several limitations to our study. First, this was a retrospective single‑center study, which causes a potential bias due to allocation based in RRF. Second, the sample size is small. Third, in the incremental group, there was a presumable influence of better GFR and urinary output on phosphate control and solute removal and not only the incremental approach itself. Fourth, some variables with potential impact on technique survival, such as glucose exposure, PTH and peritoneal protein clearance, were not recorded. Additionally, other variables could enrich our study, such as measurements concerning quality of life. On the other hand, significant strengths include the high quality of our database, which allowed us to study many variables, as well as technique and patient survival and long‑term follow‑up. Despite this, we cannot exclude the possibilities of bias by PD technique and solution improvment over time.
In summary, patients on the incremental approach had lower levels of phosphatemia and higher solute removal measured by Kt/V associated with longer exchanges and lower urea distribution volume in females, but also higher GFR and UO. Finally, they had higher technique survival, lower risk of drop out to hemodialysis and lower rate of two or more peritonitis linked to fewer exchanges. We are sure that higher GFRs influenced these parameters in the incremental approach. Thus we conclude that incremental dialysis can be safe and a reasonable strategy in PD incident patients