Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.34 no.1 Lisboa mar. 2020
https://doi.org/10.32932/pjnh.2020.04.065
CASE REPORT
Sarcoidosis: a case with pulmonary, cutaneous and renal injury
Catarina Isabel Ribeiro, Susana Pereira, Ana Ventura, Clara Almeida, Clara Santos, João Carlos Fernandes
Nephrology Department, Centro Hospitalar de Vila Nova de Gaia – Espinho
ABSTRACT
Sarcoidosis is a rare multisystem disease that is characterized by the formation of inflammatory granulomatous processes. The lung is the most frequently affected organ, but kidney injury can also occur. It is a disease with a highly variable course and prognosis and non‑consensual treatment. This report describes the successful case of a patient with sarcoidosis presenting with pulmonary, cutaneous and renal injury, who presented with adequate diagnostic timing and optimal therapy response.
Keywords: kidney injury, multisystemic disease, sarcoidosis
INTRODUCTION
Sarcoidosis is a multisystemic, immune‑mediated disease of unknown etiology. It is characterized by the formation of inflammatory granulomas.1,2 The lung is the most frequently affected organ, in 90% of cases, with the appearance of bilateral diffuse pulmonary infiltrates and hilar adenopathy.3 Sarcoidosis can also affect the skin, eye, lymphatic system, heart, kidney and central nervous system.4,5,6
Its diagnosis is based on compatible clinical and imaging findings and histopathological evidence of noncaseating granulomas in the affected organs, excluding other granulomatous diseases of similar presentation.3,4,7
Despite its often benign and self‑limiting presentation, in 30% of cases this condition can also take on a progressive and severe form if not treated timely and properly. Its course and prognosis are very variable, with an early recognition of its clinical manifestations and the appropriate investigation for the correct diagnosis and therapeutic orientation essential.3,7
CASE REPORT
The authors present the case of a 52‑year‑old male with arterial hypertension polymedicated (lisinopril, amlodipine, bisoprolol and indapamide).
He had multiple painless subcutaneous nodules, on both upper limbs and back, of unknown etiology and under surveillance. No other major medical history. No current or past smoking or drinking habits.
This patient was admitted to the Nephrology Service for acute kidney injury (AKIN classification Stage‑2).
He referred asthenia and anorexia, with the notion of unquantified weight loss and persistent dry cough in the previous 6 months (coincident with subcutaneous nodules appearance). He had no dyspnea, hypersudoresis or fever.
No mention of other symptoms. No recent infectious processes and no relevant epidemiological context. No introduction of new drugs.
At hospital admission he was hypertensive (blood pressure 211/111 mmHg), tachycardic (heart rate 107 bpm), apyretic, eupneic and without pain. He had erythematous nodular lesions, nonpruritic and painless features, about 1.0 cm in diameter and with irregular and dispersed body distribution (Figure 1). The remaining exam was normal.

The analytical study performed documented serum creatinine of 5.44 mg/dL, urea 144 mg/dL and hemoglobin 10.6 g/dL. Corrected calcium was 14.0 mg/dL (calcium ionized 1.87 mmol/L), 1,25‑dihydroxyvitamin D was 34 mmol/L, phosphorus was 3.5 mg/dL and parathyroid hormone was suppressed (3.0 pg/mL). No elevation of inflammatory parameters. Urine sediment had leukocyte 2‑5/field and no erythrocytes. Protein‑creatinine ratio from a single urine sample was 0.54. Renal tract ultrasonography presented normal kidney dimensions, with regular contours and preserved parenchymal thickness. No signs of obstructive uropathy or organized liquid collections or expansive lesions. Nephrolithiasis and nephrocalcinosis were not identified. The patient started treatment for hypercalcemia (intravenous fluid therapy and zoledronic acid), with gradual improvement of serum calcium levels and renal function.
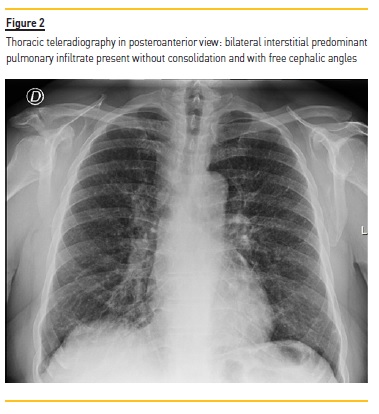
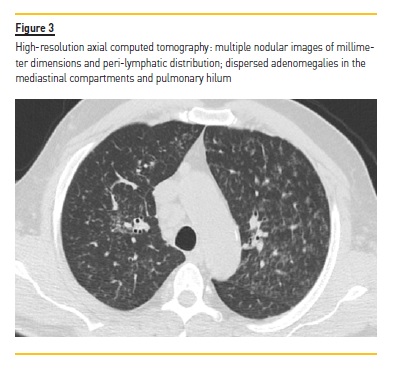
From the complementary study performed during hospital stay, thoracic teleradiography showed a bilateral interstitial infiltrate (Figure 2), later characterized by high‑resolution computed tomography, which documented numerous millimeter nodules of peri‑lymphatic distribution, with multiple adenomegalies scattered throughout the mediastinal compartments and pulmonary hilum (Figure 3).
The patient underwent bronchofibroscopy with bronchial biopsies, whose histology suggested non‑necrotizing granulomatous inflammation.
Bronchoalveolar lavage examination showed normal total cell count and differential cell count with intense lymphocytosis. Flow cytometry study of lymphocyte subpopulations revealed a high CD4/CD8 ratio (2,9). Cytology analysis was negative for malignant lesions and bacteriological examination was not viable due to contamination.
Skin biopsy documented numerous epithelioid granulomas with multinucleated giant cells, also favoring the hypothesis of chronic granulomatous inflammatory process.
The remaining study, including protein electrophoresis serum immunoglobulins and immunofixation were normal. Angiotensin converting enzyme was normal (5 U/L). Anti‑nuclear antibodies, double‑stranded DNA and anti‑neutrophil cytoplasmic antibody were negative.
Serology test for human immunodeficiency virus, B and C hepatitis, syphilis, herpes 1 and 2, Epstein‑Barr virus and cytomegalovirus were negative. DNA research of Mycobacterium tuberculosis complex was negative too.
Therefore, the presumptive diagnosis of sarcoidosis with pulmonary, cutaneous and renal involvement was assumed. The patient started oral corticosteroid therapy with prednisolone 60 mg/day and he presented excellent clinical and analytical response. He was discharged at the end of 1 week, under weaning corticosteroid and outside follow‑up.
The patient maintained full corticosteroid dose for 2 weeks, followed by 10 mg/day reduction every 2 weeks until 40 mg/day, followed by 5 mg/day reduction every 2 weeks until 10 mg/day and lastly followed by 2.5 mg/day reduction every 2 weeks until 5 mg/day. Three months later, he presented calcium levels normalization and 6 months later complete recovery of renal function. On 2‑year follow‑up, the patient continued to have normal renal function and calcium levels (serum creatinine 0.78 mg/dL and corrected calcium 9.1 mg/dL), with normal urinalysis too.
DISCUSSION AND CONCLUSION
Sarcoidosis is a rare disease with an estimated prevalence of approximately 20 per 100.000 in the caucasian population. This can affect patients of all racial backgrounds, with a similar incidence in both males and females and with an average age at presentation of approximately 50‑years‑old, as in the present case.1
Sarcoidosis is a pathology that leads to an important variability of clinical presentation. For its diagnosis, in addition to a high degree of suspicion, evidence of granulomatous inflammation in one or more organs is required, often with tissue histopathological confirmation.3,4
Typically sarcoidosis presents with the following abnormalities: bilateral hilar adenopathy, pulmonary reticular opacities and skin, joint, and/or eye lesions. Skin involvement is described in about 25% of patients,4,5 often presenting as in this case as an early finding.
The incidence of kidney disease ranges from 7 to 17% and the main manifestations include abnormal calcium metabolism, with nephrolithiasis and nephrocalcinosis and acute interstitial nephritis with or without granuloma formation. Renal insufficiency arises as a consequence of hypercalcemia or hypercalciuria (described in 40‑60% and 5‑10% respectively), and so its presence may be a diagnostic guide. Glomerular diseases were rarely reported.3,6 In the present case, the authors considered that the dysregulated calcium handling could have caused renal injury through afferent renal arteriolar vasoconstriction, thereby decreasing renal blood flow and glomerular filtration rate leading to the acute kidney injury.
In sarcoidosis there is a consensus that an initial diagnosis should be confirmed by biopsy, because later confirmation is often made more difficult by treatment, and the potential indications for treatment in the patient's further course may then be impossible to assess.8
The lung is the most common site of a confirmatory biopsy, because pulmonary involvement is very common and because bronchoscopy affords easy access. During bronchoscopy, bronchoalveolar lavage should be performed. Differential and immune cytology of the lavage sample typically reveals CD4 lymphocytosis and part of the sample should be sent for microbiological studies to rule out an infectious cause.9 As in the present case report, bronchoscopy with bronchoalveolar lavage was performed and flow cytometry study of lymphocyte subpopulations revealed a high CD4/CD8 ratio as expected.
Angiotensin converting enzyme and the soluble interleukin‑2 receptor are two commonly used biomarkers for sarcoidosis that are non‑specific and therefore unsuitable for confirming the diagnosis.10 The authors present a case of sarcoidosis diagnosis with a normal angiotensin converting enzyme.
Sarcoidosis has a variable clinical course and prognosis. One‑third of the patients undergo spontaneous remission. The absence of activity and prognostic markers, as well as the lack of treatment protocols, raises many doubts about the decision to treat and the optimal therapy to offer the patient.2,7
Oral corticosteroid administration is the standard treatment, even though the scientific evidence underlying it is inadequate by current standards and no treatment for sarcoidosis has yet been approved by the European regulatory authority.11 Most experts recommend that the initial treatment of sarcoidosis should be with prednisone at a dose of 0.5 mg/Kg. Oral corticosteroid should be given for at least 6 months, with a gradual reduction of the dose over the ensuing 6 months.12
The present case shows an example of success, whose clinical suspicion and timely diagnosis proved essential in the evolution and therapeutic response. This patient experienced a significant and rapid improvement in both renal function and hypercalcemia in response to therapy with prednisolone. In this case, the sarcoidosis diagnosis being already confirmed and the excellent response obtained may had made a kidney biopsy unnecessary. However, this subject is debatable.
Since diagnosis, treatment and its duration are not consensual, the authors consider that the follow‑up of this disease evolution is often the best decision predictor.
References
1. Elizabeth A, Yvette C. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018; 9(11): 227‑240 [ Links ]
2. Llanos O, Hamzeh N. Sarcoidosis. Med Clin North Am. 2019; 103(3): 527‑534 [ Links ]
3. Roberts SD, Mirowski GW, Wilkes D, et al. Sarcoidosis. Part II: extrapulmonary and systemic manifestations. J Am Acad Dermatol.2004; 51(4): 628‑630 [ Links ]
4. Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015; 33(3): 389‑416 [ Links ]
5. Ungprasert P, Crowson CS, Matteson EL. Influence of gender on epidemiology and clinical manifestations of sarcoidosis: a population‑based retrospective cohort study 1976‑2013. Lung 2017; 195:87. [ Links ]
6. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis. 2006; 48(5): 856‑870 [ Links ]
7. Judson MA. The diagnosis of sarcoidosis. Clin Chest Med. 2008; 29(3): 415‑427 [ Links ]
8. American Thoracic Society. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society and the World Association of Sarcoidosis and Other Granulomatous Disorders adopted by the ATS Board of Directors and by the ERS Executive Committee, Feb 1999. Am J Respir Crit Care Med. 1999; 160: 736‑755 [ Links ]
9. Costabel U, Bonella F, Ohshimo S, Guzman J. Diagnostic modalities in sarcoidosis: BAL, EBUS, and PET. Semin Respir Crit Care Med. 2010; 31: 404‑408 [ Links ]
10. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003; 123: 406‑412 [ Links ]
11. Grutters JC, van den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J. 2006; 28: 627‑636 [ Links ]
12. Judson MA. The treatment of pulmonary sarcoidosis. Respir Med. 2012; 106:1351‑1361 [ Links ]
Catarina Isabel Ribeiro, MD
Nephrology Department, Centro Hospitalar de Vila Nova de Gaia – Espinho
Rua da Palmeira, nº 147 1º esquerdo, Laborim
4430‑163 Vila Nova de Gaia, Porto – Portugal
E‑mail: catarina.isabel.ribeiro@gmail.com
ORCID: 0000‑0001‑6813‑0336
Disclosure of potential conflicts of interest:none declared
Received for publication: Oct 20, 2019
Accepted in revised form: Feb 27, 2020














