Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.33 no.3 Lisboa Sept. 2019
https://doi.org/10.32932/pjnh.2019.10.038
CASE REPORT
Fanconi syndrome related to zoledronic acid
Margarida Oliveira1, M. Teresa Santos2, Ana Costa1
1 Department of Medicine, Hospital Pedro Hispano, Matosinhos Local Health Unit, Matosinhos, Portugal
2 Nephrology Unit, Hospital Pedro Hispano, Matosinhos Local Health Unit, Matosinhos, Portugal
Abstract:
Fanconi syndrome is characterized by proximal tubular dysfunction, with inability to reabsorb bicarbonate causing type 2 renal tubular acidosis associated with aminoaciduria, normoglycemic glycosuria, hypophosphatemia with phosphaturia and proteinuria.
A 68-year-old man with a history of type 2 diabetes mellitus, high-grade papillary carcinoma of the bladder, submitted to transurethral resection in 2011 and 2013 had prostatic adenocarcinoma diagnosed in 2015 with bone metastasis under zoledronic acid. The patient was referred to the emergency department because of anemia de novo and acute on chronic kidney injury. Urinary sediment showed nonnephrotic proteinuria and renal ultrasound was normal. He had hyperchloremic metabolic acidosis with hypokalemia, hypophosphatemia, hypocalcemia, hypouricemia, without glycosuria. No evidence of hemolysis nor abnormalities in serum and urinary immunoelectrophoresis, endoscopic studies, myelogram, or bone marrow biopsy. Renal biopsy was compatible with acute tubular necrosis superimposed on diabetic and hypertensive nephropathy. Zoledronic acid was suspended and we observed slow improvement of renal function and resolution of metabolic acidosis and ionic disorders.
Zoledronic acid is nephrotoxic and may induce tubular dysfunction, which can cause Fanconi syndrome. Since 2012, there have been cases relating the administration of zoledronic acid for bone metastasis or hypercalcemia associated with malignant neoplasia with Fanconi syndrome. Although it is a rare association, it is a potentially fatal complication and renal function monitoring is essential.
Key words: Fanconi Syndrome, Nephrotoxicity, Renal tubular acidosis, Zoledronic Acid
INTRODUCTION
Metabolic acidosis can result either from the gain of endogenous or exogenous acids or from the loss of bases through a gastrointestinal or genitourinary route. Renal tubular acidosis (RTA) results from the inability of the kidney to excrete acids or to reabsorb bases, resulting in hyperchloremic metabolic acidosis (with normal anion gap)1. Type 2 (or proximal) renal tubular acidosis results from a bicarbonate resorption defect that may occur alone or more often occurs in the context of a global proximal renal tubule dysfunction known as Fanconi syndrome (FS)1,2.
Most of the plasma is filtered in the glomerulus and reabsorbed in the proximal contoured tubule. Solutes such as amino acids, phosphate and glucose enter the cell through the apical membrane of the proximal tubule through a Na+ co-transporter. Sodium transport is potentiated by the Na+/ K+ ATPase in the basal membrane of the cell that leads to Na+ absorption and its intracellular decrease3.
Proximal tubular dysfunction leads to urinary loss of substances that are predominantly or exclusively reabsorbed in this segment, namely amino acids, low molecular weight proteins, phosphate, bicarbonate, glucose and uric acid4. This leads to the features of FS4. The proximal tubule also reabsorbs a significant portion of sodium, potassium, chloride, magnesium and calcium4. Thus, FS is characterized not only by hyperchloremic metabolic acidosis but also hypokalemia, aminoaciduria, proteinuria (mainly low molecular weight proteins), phosphaturia, glycosuria. Other manifestations are hypouricemia, hyponatraemia, hypercalciuria and polyuria5. Some patients appear to have only some of the characteristics of Fanconi syndrome4, such as isolated tubular proteinuria. FS may or may not be associated with glomerular disease, and there may be severe tubular dysfunction without worsening of the glomerular filtration rate4.
The causes of FS range from hereditary diseases to acquired causes5. Hereditary diseases include cystinosis, mitochondrial cytopathy, tyrosinemia, fructose intolerance, galactosemia, Dent’s disease, Lowe’s syndrome and Wilson’s disease4,5. Among the acquired causes, the most common is pharmacological toxicity. Other causes of acquired FS are Sjögren’s syndrome, amyloidosis, multiple myeloma, monoclonal gammopathy, and light chain deposition disease and obstructive uropathy6.
CASE REPORT
The authors present the case of a 68-year-old man with type 2 diabetes mellitus diagnosed in 2003 under oral antidiabetic drugs, HbA1C 6.6% and microalbuminuria (110mg/g), no diabetic retinopathy, dyslipidemia, hypertension, chronic renal disease (which has not been previously studied), with a glomerular filtration rate (GFR) of 58ml/min/ 1.73m2 estimated by the CKD-EPI formula (stage 3a) with albuminuria 110mg/g (A2) in 2015. He had documented normal renal function before 2010 and no history of deafness. The patient had high grade papillary carcinoma of the bladder, submitted to transurethral resection in 2011 and 2013 and prostate adenocarcinoma (Gleason 4+3) with bone metastasis, diagnosed in February 2015 and since then under triptorelin, cyproterone acetate and zolendronic acid 4mg (1 intravenous administration for 15 minutes, every 4 weeks).
On April 2016, he was referred to the Emergency Department by his primary health care physician due to asthenia, anorexia and anemia de novo (last normal value in 2015). On objective examination he had mucocutaneous pallor, without any other abnormal findings. The analytical study (Table I) showed normocytic and normochromic anemia (hemoglobin 6.9g/dL) and acute on chronic kidney injury AKIN 2 (plasma creatinine 2.7mg/dL, for a previous value of 1.3mg/dL in 2015). Urinalysis with proteinuria (110mg/dL), without any other abnormal findings.
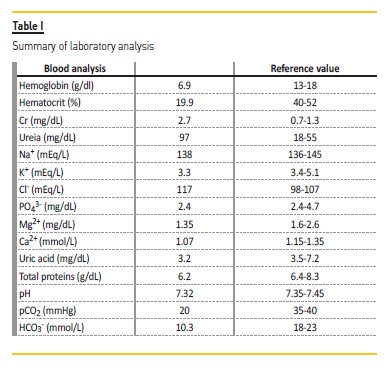
Renal ultrasound showed no abnormalities in renal or urinary tract structure. Nevertheless, he had compensated hyperchloremic metabolic acidosis (pH 7.32, pCO2 20mmHg, HCO3- 10.3mmol/L, Cl- 117mmol/L), urine pH 6.5 and hypokalemia (3.3mmol/L), hypocalcemia (1.07mg/L), hypomagnesemia (1.35mg/dL) and hypophosphatemia (2.4mg/dL).
The patient had no history of diarrhea, so the main hypothesis was that of renal tubular acidosis justifying hyperchloremic metabolic acidosis and hypokalemia. Urine analysis showed an increased bicarbonate excretion fraction of 16.4% (for a normal range <15%), supporting the diagnosis of renal tubular acidosis type 2.
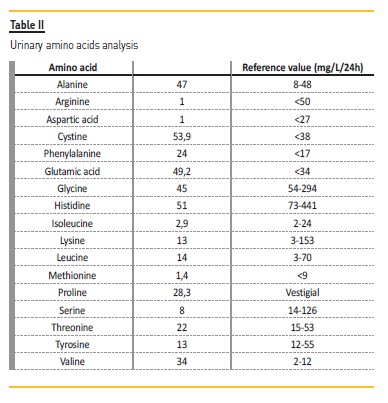
Urinary amino acids analysis (Table II) showed an increase of 5 of 17 measured amino acids, non-selective proteinuria, hypophosphatemia with phosphaturia, hypocalcemia with calciuria, hypokalemia with kaliuria and hypouricemia with uricosuria, without glycosuria. Thus, we concluded we were facing a proximal tubular dysfunction as in FS.
Considering the patient had acute on chronic kidney injury and anemia, it was mandatory to suspect f multiple myeloma, light-chain disease or amyloidosis. To complete the study, we found a low reticulocyte index (0.64%), with a slight deficit of vitamin B12 (183pg/mL), without any change in iron kinetics or folate deficiency. No changes were also found in serum and urinary immunoelectrophoresis. Myelogram showed hypocellularity with 1% plasma cells, with no qualitative abnormalities, and bone marrow biopsy showed no qualitative nor quantitative deviations nor any sign of malignancy or amyloid deposits.
Upper and lower digestive endoscopy were normal with no amyloid deposits in rectal fat biopsy. Hence, there was no evidence of any of the postulated diagnostic hypotheses. We extended the study to autoimmune paraneoplastic causes, but ANA, anti-dsDNA, anti-SSA and anti-SSB came out negative. We documented vitamin D deficiency (9U/L), but it was not possible to establish it as a cause or an effect of the disease. Renal biopsy (Fig. 1-4) was performed on 04/29/2016, showing acute tubular necrosis superimposed on diabetic nephropathy without significant expression at glomerular level and vascular lesions suggestive of hypertensive nephropathy.
In view of these findings, the patient’s previous susceptibility to renal damage due to diabetic and hypertensive nephropathy and having excluded other causes, we considered the diagnosis of zoledronic acid nephrotoxicity, and, once the zoledronic acid was suspended, the patient showed a progressive, but slow, improvement in tubular function. In 2 months, we observed the complete resolution of metabolic acidosis and ionic disorders with no need of supplementation, but the improvement in glomerular filtration rate was much slower. On January 2018, the patient had for the first time a plasma creatinine of 1.5mg/dL, with an estimated GFR of 45ml /min/1.73m2.
DISCUSSION
Zoledronic acid is a nitrogenated bisphosphonate with a half-life of 150-200 days and a low binding rate to plasma proteins (56%)7,8. It inhibits bone resorption in patients with post-menopausal osteoporosis, hypercalcemia due to malignancy, Paget’s disease and bone metastases associated with solid tumors and multiple myeloma5,8,9.
Nephrotoxicity is described for all the bisphosphonate class, but it is more frequent with higher doses and potency (namely pamidronate and zoledronic acid) and with intravenously administration8.
When administered intravenously, biphosphonates are not metabolized, and are excreted unaltered by the kidneys7,10 through glomerular filtration8 and a small percentage is actively secreted at the tubular level. As a result, when the renal function is impaired, bisphosphonate excretion is reduced which can lead to excessive serum and bone levels resulting in toxicity8. Bisphosphonate nephrotoxicity is both dose-dependent and infusion time-dependent8. Patterns in renal biopsies include acute tubular necrosis, segmental and focal glomerulosclerosis and a case of acute interstitial nephritis was described8-10.
Acute tubular necrosis, the main pattern of injury seen in patients with zoledronic acid nephrotoxicity, is characterized by toxicity directed at tubular epithelium8. This toxicity can be expressed in a number of ways, namely by reducing the permeability of the apical membrane, by failure of endocytosis and apical transporters, by lysosomal dysfunction, by glutathione depletion, by mitochondrial toxicity and consequently a decrease in ATP production or by direct inhibition of ATPase Na+/K+4,8. It is believed that bisphosphonate nephrotoxicity results from direct damage to the tubular cells by inducing apoptosis through the mevalonate pathway, in a similar way to its apoptotic effect on osteoclasts8,9.
The estimated incidence of acute tubular necrosis associated to zoledronic acid is 2-10%9. In a 2003 study published in the NEJM10, 72 cases were identified by the FDA between 2001 and 2003 of zoledronic acid-associated renal dysfunction, with 6 of these referring to patients with prostate cancer10. The mean time from onset of treatment to the detection of renal failure was 56 days (1-242 days), with a mean of 2.4 administrations. In this series10, 48 patients required hospitalization, 27 patients needed dialysis and 18 died. The majority of patients had a baseline creatinine level of 1.4-3mg/dL, with a mean exacerbation up to 6.5mg/dL during treatment with zoledronic acid and recovery to 2.7mg/dL after discontinuation of the drug. In this case, the time from onset of administration to the onset of the first symptoms was about 365 days, with a cumulative dose of 40mg, with a previous creatinine of 1.3mg/dL and a maximum of 3.3mg/dL and recovery to 1.5mg/dL after discontinuation of the drug. In all the reported cases, the treatment is the suspension of the nephrotoxic drug and the ionic supplementation as required, as in the present case.
While acute kidney injury is infrequent, FS is quite rare. FS seems to be the result of an overall dysfunction of solute transport at the proximal tubule level4. It is believed that the incidence of renal tubular toxicity is underestimated by the absence of systematic studies to monitor tubular dysfunction (and not only a decrease in GFR) and underreporting of cases4. Although the renal tubules have a great regenerative capacity, this can take months and it may not always be complete, leading to chronic disfunction of renal tubules even after the withdrawal of the drug4. The first reported case of FS related to zoledronic acid was in 2012. Since then, there have been other cases in which the administration of zoledronic acid was associated with serum and urinary electrolyte abnormalities compatible with FS, improving after drug withdrawal.
This case brings together several of Naranjo’s criteria for adverse drug reaction, namely: the occurrence of the adverse event after exposure to the drug, improvement after discontinuation of the drug, lack of a better diagnostic explanation and the existence of other scientific reports documenting this association11.
The authors note that zoledronic acid has been shown to be of benefit in patients with bone metastasis, with a well-established safety profile when used properly10. Its nephrotoxic potential does not contraindicate its use, especially in cases where the benefits outweigh the risks4. The Food and Drug Administration recommends monitoring serum creatinine prior to each treatment and adjusting doses in patients with chronic kidney disease and treatment should be withheld when there is an increase of 0.5mg/dL in patients with previous normal renal function or 1mg/dL in patients with previous abnormal renal function8. Nevertheless, the European Commission permits renal function to be monitored at the discretion of the physician8. It is also recommended to respect infusion time8. We should note that despite the patient’s GFR being 58ml/min/1.73m2 in 2015, zoledronic acid was administered in full dose and no monitoring of creatinine clearance was made before each dose.
We emphasize it is essential to monitor renal function and ensure adequate hydration, with special attention being paid to patients with diabetes mellitus and hypertension, as was the case with this patient (although hypertension was not previously diagnosed or treated).
Since there is not always a decrease in the rate of glomerular filtration associated with FS, monitoring of proximal tubular function during therapy with zoledronic acid is also recommended4,5.
References
1. Soleimani M, Rastegar A. Pathophysiology of renal tubular acidosis: Core Curriculum 2016. Am J Kidney Dis. 2016;68(3):488-498 [ Links ]
2. Perrone D, Afridi F, King-Morris K, Komarla A, Kar P. Proximal renal tubular acisosis (Fanconi Syndrome) induced by apremilast: A case report. Am J Kidney Dis. 2017;70(5):729-731 [ Links ]
3. Possible Fanconi Syndrome associated with zoledronic acid. Available at: http://www.hsa.gov.sg/content/hsa/en/Helth_Products_Regulation/Safety_Information_and_Product_Recalls/Product_Safety_Alerts/2015/possible-fanconisyndromeassociatedwithzoledronicacid.html. Accessed May 3, 2016 [ Links ]
4. Hall AM, Bass P, Unwin RJ. Drug-induced renal Fanconi Syndrome. QJM. 2014;107(4):261-269 [ Links ]
5. Yoshinami T, Yagi T, Sakai D, Sugimoto N, Imamura F. A case of acquired Fanconi Syndrome induced by zoledronic acid. Intern Med. 2011;50(9):1075-1079 [ Links ]
6. Yaxley J, Pirrone C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J. 2016;16(4):525-530 [ Links ]
7. Gutiérrez Sánchez MJ, Petkov Stoyano V, Pedraza Cezón L, Martín Navarro JA, Insuficiencia renal por nefropatia tubulointersticial com tubulopatía proximal tipo Fanconi tras tratamento com ácido zoledrónico. Nefrología. 2017;37:660-661 [ Links ]
8. Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74(11):1385-1393 [ Links ]
9. Torimoto K, Okada Y, Arao T, Mori H, Tanaka Y. A case of zoledronate-induced tubulointerstitial nephritis with Fanconi Syndrome. Endocr J. 2012;59(12):1051-1056 [ Links ]
10. Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349(17):1676-1679 [ Links ]
11. Naranjo CA et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239245 [ Links ]
Margarida Oliveira, MD
Department of Medicine, Hospital Pedro Hispano
Rua Dr. Eduardo Torres, 4464-513 Senhora da Hora, Matosinhos, Portugal
E-mail: med6084@ulsm.min-saude.pt
Acknowledgments
To Professor Rui Henrique for kindly providing the renal biopsyimages.
Disclosure of potential conflicts of interest: none declared
Received for publication: Sep 12, 2019
Accepted in revised form: Sep 26, 2019














