Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.4 Lisboa Dec. 2018
CASE REPORT
Therapeutic plasma exchange in the treatment of severe hypertriglyceridemia: a case report
Tiago J Carvalho, Ana Rita Martins, Rita Calça, João Sousa Torres, Célia Gil, Ilídio Rodrigues, Maria Augusta Gaspar, Teresa Adragão, Margarida Bruges
Nephrology Department, Hospital de Santa Cruz, Centro Hospitalar Lisboa Ocidental, Lisboa, Portugal
ABSTRACT
Severe hypertriglyceridemia is defined as a serum triglyceride level >885mg/dl. It is an uncommon condition associated with serious complications, mainly acute pancreatitis. Treatment includes lifestyle changes, medical therapy and, more recently, extracorporeal apheresis techniques. Herein, we report a case of severe hypertriglyceridemia managed with therapeutic plasma exchange. Our patient presented with severe upper abdominal pain. Bloodwork showed extremely severe hypertriglyceridemia (14871mg/dl) and hypercholesterolemia (1536mg/dl) with only slight elevation of amylase and lipase and no echographic signs of acute pancreatitis. Two sessions of therapeutic plasma exchange were performed, with a mean 3L of plasma treated per session.
The triglyceride levels dropped to 2325mg/dl after the first session and to 1207mg/dl after the second. Medical therapy was started, and the patient was discharged a week later. After 36 months of follow‑up she remains stable, with triglyceride levels 427mg/dl. In this case therapeutic plasma exchange was an effective and well tolerated treatment for severe hypertriglyceridemia.
Keywords: Hypertriglyceridemia, Therapeutic Plasma Exchange, Abdominal Pain
INTRODUCTION
Hypertriglyceridemia (HTG) is defined as a serum triglyceride (TG) level >150mg/dl. It is classified as severe if the TG level is >885mg/dl1. Severe HTG is an uncommon condition that affects 0,4% of adults2.
It can have several causes: (i) primary (Frederickson classification dyslipidemias I, IV and V); (ii) secondary to acquired conditions (obesity, metabolic syndrome, uncontrolled type 1 and 2 diabetes mellitus, chronic kidney disease, hypothyroidism, nephrotic syndrome, pregnancy, alcoholism, auto‑immune diseases and drugs such as corticosteroids, thiazide diuretics, beta‑blockers, estrogen, tamoxifen and atypical anti‑psychotics, among others); (iii) a combination of underlying predisposition aggravated by secondary causes3.
Severe HTG is associated with serious complications, including an elevated risk of acute pancreatitis, fatty liver disease and cardiovascular events4. Currently, up to 10% of all pancreatitis episodes are estimated to be due to HTG5. Patients with severe HTG can present with abdominal, lumbar or thoracic pain, nausea, vomiting and shortness of breath. However, they can also be asymptomatic. On physical examination hepatomegaly, xanthomas, xanthelasmas and lipaema retinalis may be evident.
Treatment of HTG includes (i) lifestyle changes, such as dietary intervention (avoidance of simple carbohydrates, adoption of a low‑fat, fiber‑rich diet and no alcohol consumption), weight loss, physical activity and smoking cessation, and (ii) pharmacologic therapy, including fibrates, omega‑3 fatty acids and niacin. In acute cases heparin and insulin may also be useful due to their stimulatory effect in lipoprotein lipase activity. These measures are effective and proven to be safe3,5,6.
The removal of triglyceride‑rich lipoproteins by extracorporeal apheresis has been shown in several case series and case reports to be effective in patients with severe HTG complicated by acute pancreatitis or refractory to optimized conventional therapy4,7‑10.
We report a case of severe HTG that was successfully treated with therapeutic plasma exchange (TPE) combined with medical therapy.
Case Report
43‑year‑old white female with (i) primary focal and segmental glomerulosclerosis diagnosed six months prior to hospital admission and medicated with prednisolone, currently 15mg/day; (ii) obesity (body mass index 31kg/m2); (iii) mixed dyslipidemia diagnosed at age 12 and treated with statin and fibrate; (iv) insulin‑dependent type 2 diabetes mellitus; (v) arterial hypertension treated with perindopril, diltiazem and carvedilol; (vi) active smoking habits (35 pack‑years) and (vii) a previous episode of alithiasic necrotizing acute pancreatitis 5 years ago. Adherence to medical therapy was irregular. The last glycated hemoglobin value before hospital admission was 10%. There was no known family history of hereditary diseases, namely familial forms of dyslipidemia.
She presented to the emergency department with severe upper abdominal pain which radiated to the back, with no other associated symptoms. The physical examination was notable for upper abdominal pain on palpation with no signs of peritoneal irritation and a full moon facies. Bloodwork showed severe hypertriglyceridemia (14871mg/dl), hypercholesterolemia (1536mg/dl) and hyperglycemia (616mg/dl). Amylase and lipase were slightly elevated (115U/L and 454U/L, respectively). Liver function tests were normal (AST 32U/L, ALT 37U/L, GGT 33U/L), as was serum creatinine (0.83mg/dl). The remaining bloodwork was unremarkable.
An abdominal ultrasound was performed, which revealed hepatic steatosis and no signs suggestive of acute pancreatitis. An insulin perfusion was started to achieve glycemic control and the patient was transferred to the nephrology department.
Considering the acute, symptomatic severe hypertriglyceridemia, the patient was treated with TPE. The treatment was performed using a PrismaFlex® monitor with a TPE 2000® filter (Gambro/Baxter Int., Lund, Sweden).
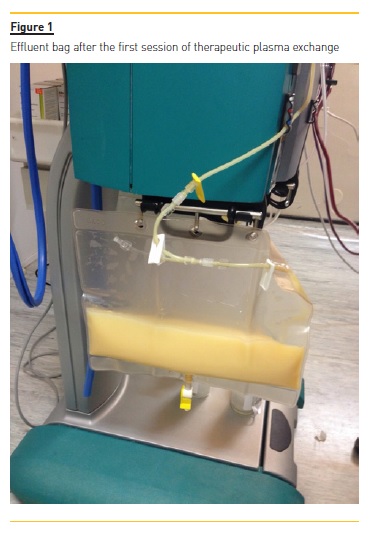
The vascular access was a central venous catheter placed on the right internal jugular vein. The replacement fluid was 5% albumin and unfractionated heparin was used as anticoagulation. Plasma volume to treat was calculated using the Kaplan formula, with a mean of 3L treated per session (one plasma volume). A total of two sessions were performed, 36 hours and 60 hours after presentation, respectively. They were well tolerated, without any significant adverse effects. The effluent bag after the first TPE session is shown in Figure 1, highlighting the lipemia.
The TG level dropped to 2325mg/dl after the first session and to 1207mg/dl after the second session.
Serum cholesterol level normalized (150mg/dl). Fenofibrate 267mg/day was started. The patient remained in the hospital for a week, with additional decline in TG levels. She was discharged with TG 941mg/dl, referred to Nephrology and Endocrinology consultations and encouraged to stop smoking, adopt an active lifestyle and comply with dietary and therapeutic recommendations. The evolution of lipid levels during the hospitalization period is shown in Table I.
After three years of follow‑up the patient remains clinically stable. Prednisolone dose was reduced to 5mg/day but not stopped due to sustained proteinuria between 2‑3g/day. Fibrate therapy was maintained and atorvastatin 20mg and omega‑3 fatty acids 3g/day were started to optimize lipid control. The patient complied with medical therapy but maintained a sedentary lifestyle and continued to smoke. The evolution of TG and total serum cholesterol, LDL‑cholesterol, HDL‑cholesterol and glycated hemoglobin levels after discharge is shown in Table II. There were no new hospitalizations nor need for additional TPE sessions.
DISCUSSION AND CONCLUSIONS
Severe HTG is an uncommon condition that can lead to severe complications, mainly acute pancreatitis. In such cases, a rapid decrease of TG levels is crucial to the management of the patient5. Heparin and insulin stimulate lipoprotein lipase activity and reduce TG levels.
However, their efficacy is not well established in very severe HTG or HTG‑induced acute pancreatitis and they may be insufficient to obtain a rapid response11.
TPE is an extracorporeal technique performed using a large pore membrane to remove substances with a high molecular weight from circulation. It consists in removing plasma and exchanging it for a replacement fluid such as albumin, plasma or a mix of albumin and saline solutions. It is a nonselective technique, as all plasma components are discarded (lipoproteins, albumin, clotting factors and immunoglobulins). The most common complications of TPE are urticaria, paresthesia, headache, muscle cramps, hypotension, hypocalcemia, bleeding and infection (both catheter‑associated and related to immunoglobulin depletion)12.
TPE has been shown in several case series and case reports to be effective in patients with severe HTG complicated by acute pancreatitis or refractory to optimized conventional therapy4,7‑10.
In our case, the patient was symptomatic and, although she showed no significantly elevated amylase or lipase levels, their measurement may be impaired by severe HTG, so acute pancreatitis was possible. It was decided not to perform a CT scan because a rapid lowering of TG levels was deemed necessary whether pancreatitis was present or not to prevent further complications. Thus, the patient underwent TPE. One plasma volume exchange was performed in each session (approximately 3L of plasma), according to the data reported in a multicenter study by Stefanutti et al10. This treatment had a remarkable efficacy, lowering TG levels by 84.4% after one session and 91.8% after two sessions. These numbers are similar to those reported in a recent systematic review by Click et al. which showed an average reduction of TG levels by 85.4%13. The decision not to perform additional TPE sessions was made on the basis that the patient was rendered symptom‑free and that the TG levels had dropped over 90% and continued to drop after the second session with medical therapy alone, with no rebound after stopping TPE.
According to the American Society of Apheresis guidelines, HTG is a class III indication for TPE, which means that the optimum role of apheresis therapy is not established, and decision making should be individualized.
In our case TPE was a safe and efficacious technique in the acute management of severe HTG, similar to several previous reports4,7‑10,14,15.
Concurrently with providing acute treatment and stabilizing the patient with severe HTG, it is imperative to establish the etiology of the HTG and correct the precipitating factors. Chylomicronemia (based on serum TG levels >885mg/dl) can be primary or secondary.
Genetic predisposition includes rare monogenic autosomal recessive forms, with mutations predominantly in the LPL gene or in genes that encode interacting factors, including APOA5, APOC2, LMF1 and GPIHBP116,17.
More commonly, polygenic factors are involved. These are multiple genetic variants with individually small effects on TG levels but that, cumulatively, increase the risk of developing chylomicronemia16.
However, secondary factors are usually required to develop this syndrome. These include uncontrolled diabetes mellitus, alcoholism, obesity, nephrotic syndrome, drugs (namely, beta blockers, estrogen, tamoxifen and corticosteroids), among others3,18,19. In this case, several reasons for the decompensation were present: uncontrolled diabetes, treatment with corticosteroids, concomitant focal and segmental glomerulosclerosis with proteinuria, poor adherence to therapy, sedentary lifestyle and obesity. Additionally, the diagnosis of dyslipidemia at age 12 suggests that a genetic predisposition may be present, despite the absence of family history of HTG. However, since genetic testing was not performed this could not be ascertained.
After hospital discharge the patient continued to have elevated levels of both fasting TG and total cholesterol in the following 36 months, ranging from 318‑861mg/dl and 289‑440mg/dl, respectively. The ongoing sedentary lifestyle combined with diabetes mellitus, obesity, uncontrolled proteinuria (2‑3g/day) and prednisolone therapy (despite the low dose) may explain these values2,3. However, the compliance with medical therapy and dietary changes have so far prevented another acute exacerbation like the one described here.
Interestingly, this patient had a previous episode of alithiasic necrotizing acute pancreatitis. Although no etiology was established at that time, it is very likely that the cause was severe HTG. Also, the pancreatic damage sustained in that episode probably resulted in the minor increases of lipase and amylase in the currently reported case despite very high TG levels.
In conclusion, in this case TPE was an effective, rapid and well tolerated treatment, with no complications noted. However, randomized controlled trials are warranted to further access the efficacy of TPE as a treatment modality for HTG and HTG‑associated pancreatitis.
References
1. Hegele RA, Ginsberg HN, Chapman MJ et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol.2014;2(8):655-66. [ Links ]
2. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169:572. [ Links ]
3. Berglund L, Brunzell JD, Goldberg AC et al. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-89. [ Links ]
4. Carrillo IG, Demelo‑Rodriguez P, Ferrero ML, Anaya F. Double filtration plasmapheresis in the treatment of pancreatitis due to severe hypertriglyceridemia. J Clin Lipidol. 2015;9(5):698-702. [ Links ]
5. Valdivielso P, Ramirez‑Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689-94. [ Links ]
6. Chaudhary A, Iqbal U, Anwan H, Siddiqui HU, Alvi M. Acute pancreatitis secondary to severe hypertriglyceridemia: management of severe hypertriglyceridemia in emergency setting. Gastroenterol Res. 2017;10(3):190-2. [ Links ]
7. Wassay SA, Dar FJ, Saleh AK, Mansoor I. Role of therapeutic plasma exchange in the treatment of severe hypertriglyceridemia: an experience. Ther Adv Endocrinol Metab 2017;8(12):169-72. [ Links ]
8. Joglekar K, Brannick B, Kadaria D, Sodhi A. Therapeutic plasmapheresis for hypertriglyceridemia‑associated acute pancreatitis: case series and review of the literature. Ther Adv Endocrinol Metab. 2017;8(4):59-65. [ Links ]
9. Castro FS, Nascimento AM, Coutinho IA, Alcazar FR, Filho JM. Plasmapheresis as a therapeutic approach for hypertriglyceridemia‑induced acute pancreatitis. Rev Bras Ter Intensiva. 2012;24(3):302-7. [Portuguese] [ Links ]
10. Stefanutti C, Di Giacomo S, Vivenzio A et al. Therapeutic plasma exchange in patients with severe hypertriglyceridemia: a multicenter study. Artif Organs. 2009;33(12):1096-102. [ Links ]
11. Alagozlu H, Cindoruk M, Karakan T, Unal S. Heparin and insulin in the treatment of hypertriglyceridemia‑induced severe acute pancreatitis. Dig Dis Sci. 2006;51:931-3. [ Links ]
12. Szczeklik W, Wawrzycka K, Wludarczyk A et al. Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiol Intensive Ther. 2013;45:7-13. [ Links ]
13. Click B, Ketchum AM, Turner R et al. The role of apheresis in hypertriglyceridemia‑induced acute pancreatitis: a systematic review. Pancreatology. 2015;15:313-20. [ Links ]
14. Schwartz J, Winters JL, Padmanabhan A et al. Guidelines on the use of therapeutic apheresis in clinical practice‑evidence‑based approach from the Writing Committee of the American Society for Apheresis: the seventh special issue; J Clin Apheresis. 2016;31:149-338. [ Links ]
15. Seda G, Meyer JM, Amundson DE et al. Plasmapheresis in the management of severe hypertriglyceridemia. Crit Care Nurse. 2013;33:18-23; quiz 4. [ Links ]
16. Brahm AJ, Hegele RA. Chylomicronaemia – current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11:352-62. [ Links ]
17. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001. [ Links ]
18. Brunzell JD, Bierman EL. Chylomicronemia syndrome. Interaction of genetic and acquired hypertriglyceridemia. Med Clin North Am. 1982;66:455-68. [ Links ]
19. Stone NJ. Secondary causes of hyperlipidemia. Med Clin North Am. 1994;78:117–1141. [ Links ]
Tiago J Carvalho, MD
Av. Prof. Dr. Reinaldo dos Santos
2790‑134, Carnaxide
E‑mail: tiagojc18@hotmail.com
Disclosure of potential conflicts of interest: None declared.
Received for publication: Oct 21, 2018
Accepted in revised form: Dec 6, 2018














