Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.4 Lisboa Dec. 2018
CASE REPORT
Persistent knee pain in a patient with systemic lupus erythematosus
Miguel Bigotte Vieira1,*, Joana Monteiro Dias1,*, José Carlos Romeu2, Marta Pereira1, Cristina Pinto de Abreu1
1 Serviço de Nefrologia e Transplantação Renal, Centro Hospitalar Lisboa Norte, Portugal
2 Serviço de Reumatologia e Doenças Ósseas Metabólicas, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Centro Académico de Medicina de Lisboa, Portugal
* Equally contributing first authors
ABSTRACT
The evaluation of chronic kidney disease-mineral and bone disorder (CKD-MBD) is based on clinical, laboratory, radiology and histological data. However, these data may not always be entirely concordant, leading to significant doubt over therapeutic options. We present a challenging CKD-MBD case report.
A 26-year-old female patient presented a past medical history of systemic erythematosus lupus and end-stage renal disease. She had been on hemodialysis and transited to peritoneal dialysis due to multiple vascular access failure. Medication included calcium carbonate, cholecalciferol, calcitriol, and cinacalcet but adherence to medication was low, leading to PTH values often higher than 1000 pg/mL. She presented right knee pain for two months. Radiology exams revealed iliac, femoral and tibial osteolytic lesions. A bone biopsy was performed, detecting disperse multinucleated giant cells compatible with brown tumors. However, poor therapeutic adherence did not allow persistent control of hyperparathyroidism, with PTH levels often >1000 pg/mL, and parathyroidectomy was considered. A tetracycline-labeled bone biopsy showed high turnover bone disease in the past, evolving to low-bone turnover/adynamic bone disease. This led to the decision of optimizing medical treatment and avoiding parathyroidectomy. Significant clinical improvement was observed with knee pain resolution and transient reduction of PTH to stage 5 chronic kidney disease reference levels. Four years later an imagiological re-evaluation was performed, revealing bilateral tibial, femoral and iliac enlarged bone lesions, and bone scintigraphy confirmed hypermetabolic lesions. She was then submitted to subtotal parathyroidectomy and subsequently to deceased-donor renal transplantation. This case report shows the importance of closely following CKD-MBD patients, even in asymptomatic patients. Treatment decision should not solely include bone histology criteria. Repeated evaluations at different time points of clinical, laboratory, and radiology data might be particularly useful.
Keywords: Chronic kidney disease-mineral and bone disorder, hyperparathyroidism, brown tumor.
INTRODUCTION
Chronic kidney disease-mineral and bone disorder (CKD-MBD) is characterized by one or more of the following: abnormalities of calcium, phosphorus, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and vitamin D metabolism; abnormalities in bone turnover, mineralization, volume linear growth, or strength, and extraskeletal calcification.1 Renal osteodystrophy is defined as abnormal bone histology and is included in CKD-MBD bone abnormalities. The evaluation of CKDMBD is based on clinical, laboratory, radiology and histological data.2 Notably, the gold standard for the assessment of renal osteodystrophy is a bone tissue biopsy.3
This exam should be considered in patients in whom the type of renal osteodystrophy is not clear.2 However, laboratory, radiology, and histological data may not always be entirely concordant, leading to significant difficulties in establishing the predominant type of CKDMBD abnormality and, therefore, the best course of treatment. Herein, we present a CKD-MBD case report in which treatment decision was challenging.
CASE REPORT
We present a case of a 26-year-old female, previously diagnosed with systemic lupus erythematosus at the early age of 11, including multi-organ involvement (renal, hematologic, articular, and cutaneous), and secondary antiphospholipid syndrome. Additionally, the patient had a previous history of hypertension, dyslipidemia, recurrent urinary tract infections, and bilateral cataracts due to corticotherapy.
Despite aggressive therapy with multiple cycles of high dose corticosteroids, mycophenolate mofetil, cyclophosphamide and rituximab, the patient progressed to end-stage renal disease and started haemodialysis in 2009 at the age of 17. While on haemodialysis, she presented multiple vascular access thrombosis and several haemodialysis catheters were inserted, leading to inferior vena cava thrombosis and bilateral brachiocephalic trunk stenoses. Furthermore, a large compressive haematoma after vascular access puncture led to severe ischemia of the right hand. This event led to the transition to peritoneal dialysis in 2010. The patient was taking prednisolone, mycophenolic acid, enoxaparin, alfa darbepoetin, folic acid, vitamin B complex, calcium carbonate, calcitriol, cholecalciferol, cinacalcet, omeprazole, and simvastatin. The automated peritoneal dialysis protocol included 10 L of 1.36% glucose plus 2 L of icodextrin solution per day.
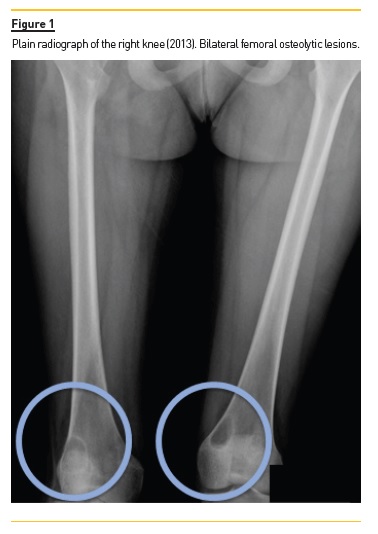
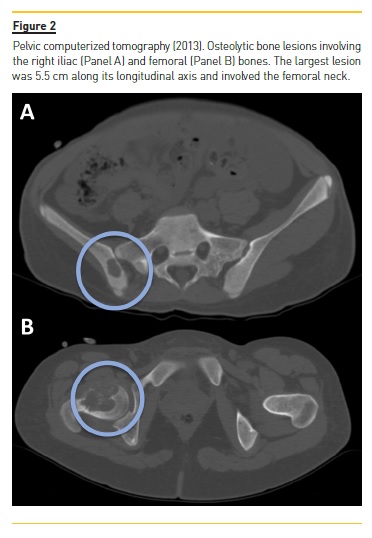
She developed CKD-MBD and maintained marginal control of PTH, calcium and phosphorus levels throughout the following years, mainly in association with low therapeutic adherence, leading to high PTH levels, frequently >1000 pg/mL. In 2013, four years after initiation of renal replacement therapy and three years after starting peritoneal dialysis, she presented right knee pain with limited range of motion which gradually worsened for two months. At that time PTH value was 1119 pg/mL. Plain radiographies revealed lytic lesions in distal femurs, proximal right femur, and right proximal tibias (Fig. 1). Computerized tomography (Fig. 2) confirmed the osteolytic bone lesions and revealed another lesion with the same features at the right iliac. The lytic lesion at right neck femur was 5.5 cm along its longitudinal axis and presented a thinned inner cortex. Computed tomography guided bone biopsy from proximal tibia was performed and documented proliferation of fibrous connective tissue and hemosiderin deposits with disperse multinucleated giant cells. These findings were compatible with brown tumors associated with severe hyperparathyroidism.
At this time, it was decided to increase medical therapy and to reinforce the importance of strict therapeutic compliance. Cinacalcet was increased to 180 mg/day resulting in rapid PTH control, which was lower than 200 pg/mL two months after therapeutic adjustment. Additionally, due to the risk of fracture associated with the lytic lesion at right neck femur, prophylactic nailing of the proximal femoral epiphysis supplemented with polymethacrylate was performed.
After six months, the patient was clinically asymptomatic, PTH remained in reference levels, and plain radiograph re-evaluation of the bone lesions revealed stable lesions. However, due to poor treatment compliance, adequate control of hyperparathyroidism was not sustained after this period, and the patient presented wide range of PTH levels, from <200 to >1000 pg/mL. Thus, parathyroidectomy was considered.
Tetracycline-labeled bone biopsy revealed preserved total bone volume, osteoid tissue surface and volume within reference limits, and reduced thickness strips, compatible with absence of mineralization defect. There were multiple past bone reabsorption scars, including Howships lacunae that had not been replaced by recent bone tissue, favoring high turnover bone disease. However, there was also absence of bone formation and reabsorption, and very scarce intertrabecular fibrosis, favoring lowbone turnover/adynamic bone disease. Thus, this result was surprisingly compatible with high turnover bone disease in the past, evolving to low-bone turnover/adynamic bone disease. Following a multidisciplinary team meeting and taking into consideration the bone histology results, it was decided not to perform a parathyroidectomy.
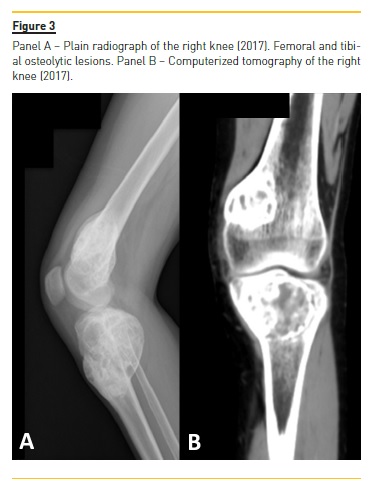
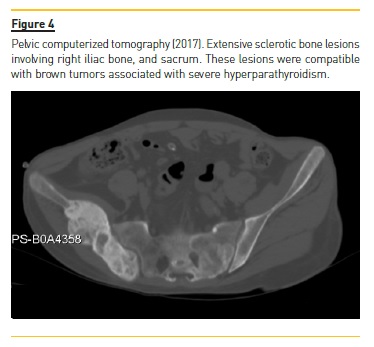
Four years later (2017), asymptomatic bone tumefactions of the knees were observed and imagiological re-evaluation was performed (Fig. 3 and 4), revealing expansion and esclerosis of previous lesions involving right iliac bone, distal femurs and right proximal tibia, as well as a new sacrum lesion. Bone scintigraphy confirmed hypermetabolic lesions and a subtotal parathyroidectomy was then performed.
The patient presented gradual reduction in urine output and became anuric. Ultrafiltration achieved in automated peritoneal dialysis with 2.27% glucose solutions and icodextrin was inferior to 700 ml per day.
Hypertension was the main sign of hypervolemia. The peritoneal equilibration test revealed ultrafiltration failure (244 ml after a four-hour dwell of a 2 L 3.86% glucose solution), impaired aquaporin channel-mediated water transport and transition to a higher membrane transport type (dialysate-to-plasma ratio [D/P] of creatinine = 0.79). As the patient presented multiple vascular access failure and ultrafiltration failure in peritoneal dialysis, she was transitioned from the regular waiting list to the urgent waiting list for renal transplantation.
The patient was submitted in the same year to deceased-donor kidney transplantation. Nine months after subtotal parathyroidectomy, the patient is asymptomatic, presents reasonable renal function with serum creatinine of 1.5 mg/dL (estimated glomerular filtration rate of 48 ml/min/1.73m2 using CKD-EPI equation), low PTH values (10 pg/mL), normal total serum calcium concentration (9.4 mg/dL), normal serum phosphorus (3.6 mg/dL), and plain radiographs re-evaluation revealed stabilization in bone lesions size.
DISCUSSION
Chronic kidney disease-mineral and bone disorder is almost universal in chronic kidney disease patients on dialysis and remains a subject of active debate regarding diagnosis and treatment. There is a large variation in PTH values between patients and the same PTH value can be associated with either adynamic or high turnover bone disease. Parathyroid hormone values also vary between races. Black patients tend to present higher PTH levels at any level of bone turnover and tend to have lower bone turnover at any level of PTH.4 Since PTH values per se are insufficient to clarify the type of renal osteodystrophy, bone biopsy is still considered the gold standard for its diagnosis.5 The 2017 CKD-MBD Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend a target value of two to nine times the normal PTH levels, reflecting a range of values in which the renal osteodystrophy risk seems to be lower.2 Although the ideal target of PTH in CKD patients is still largely unknown, levels frankly above the 2017 CKD-MBD KDIGO established target seem to frequently be associated with high turnover bone disease, whereas the reverse is true for adynamic bone disease. Although these abnormal PTH values are related to bone biopsy findings, their predictive ability to ascertain the type of renal osteodystrophy may be inaccurate and even patients with PTH levels sustained and within range may present pathologic bone biopsy findings.5 The patient presented PTH values often >1000 pg/mL when knee pain firstly appeared and osteolytic lesions were diagnosed. Nevertheless, she promptly responded to medical therapy with rapid normalization of PTH values after cinacalcet dose increase and, probably most importantly, superior therapeutic adherence. However, poor therapeutic adherence did not allow longer maintenance of adequate control of hyperparathyroidism, with PTH levels often >1000 pg/mL, and parathyroidectomy was considered.
High fluctuation of PTH levels throughout the years and presence of additional risk factors for osteoporosis including previous treatment with high dose corticosteroids led to the presumption of a multifactorial bone disease. This led to higher difficulty in estimating both evolution and prognosis. Corticosteroid therapy is commonly associated with impaired bone formation and osteoporosis and constitutes the most common cause of secondary osteoporosis. It is the most frequent cause of osteoporosis in young people.6 However, osteoporosis diagnosis is harder to establish in the setting of CKD as CKD-MBD features may predominate.
Tetracycline-labeled bone biopsy revealed preserved total bone volume, osteoid tissue surface and volume within reference limits, and reduced thickness strips compatible with absence of mineralization defect. There were multiple past bone reabsorption scars, including Howships lacunae that had not been replaced by recent bone tissue favoring high turnover bone disease.
However, there was also absence of bone formation and reabsorption, and very scarce intertrabecular fibrosis, favoring low-bone turnover/adynamic bone disease. Thus, this result was surprisingly compatible with high turnover bone disease in the past, evolving to low-bone turnover/adynamic bone disease. Although this result was not predicted, it was considered the most relevant result due to the known possible low correlation between PTH levels and type of renal osteodystrophy and the requirement of bone biopsy to establish the definitive diagnosis of renal osteodystrophy.7-9
The use of cinacalcet has previously been associated with PTH reduction, decrease in bone formation rate, erosion in bone perimeter, transition from high-turnover to normal bone histology and, in some patients, to adynamic bone disease or osteomalacia.10 We consider this fact may partially explain the findings of the biopsy. Four years later, asymptomatic enlarged bone lesions were detected on imaging reassessment and a parathyroidectomy was performed. In retrospective, it is possible to consider that a different approach could have been made at the time of diagnosis of osteolytic lesions. However, note that the patient remained asymptomatic; histology suggested evolution from high turnover bone disease to low-bone turnover/adynamic bone disease; PTH had returned to reference levels and bone lesions had been shown to be stable six months after being discovered. Thus, clinical, laboratory, radiology and histological data were not entirely concordant and were unable to predict the clinical outcome years later.
In the management of secondary hyperparathyroidism, parathyroidectomy is usually performed when hyperparathyroidism is refractory to medical therapy or when maximum dose is not possible due to medication side effects.9 Treatment with vitamin D analogs may be constrained by hypercalcemia, hyperphosphatemia, and/or parathyroid gland resistance whereas treatment with calcimimetics may be curbed by hypocalcemia or gastrointestinal side effects. Large observational dialysis cohorts have shown positive associations between parathyroidectomy and decreased all-cause mortality, normalization of calcium and phosphorus, decreased fracture rate, and improved healthrelated quality of life. After 5–10 years on dialysis, this procedure will have been performed in about 15% of patients. Persistently elevated PTH values >800 pg/ml for more than 6 months despite maximal management with vitamin D analogs and calcimimetics are generally accepted as an indication for parathyroidectomy.
Although there is no definitive lower PTH threshold, parathyroidectomy in patients on dialysis is reasonable when PTH levels are between 600 and 800 pg/ml if there is persistent hypercalcemia or hyperphosphatemia, elevated risk or presence of calciphylaxis, or erythropoietin-resistant anemia. There are no significant differences in surgical outcomes between subtotal and total parathyroidectomy. In subtotal parathyroidectomy, a fragment of parathyroid tissue is placed into the sternocleidomastoid or forearm muscle or the subcutaneous abdominal adipose tissue. Thus, it presents a lower risk of postoperative hypocalcemia due to hungry bone syndrome.
In conclusion, this case report not only shows the difficulty in establishing the best course of treatment in CKD-MBD patients, but also reinforces the need to frequently reassess the patients in which the available data are not consistent. Treatment decision should not solely include bone histology criteria. Repeated measurements at different time points of clinical, laboratory, radiology, and bone biopsies data might be particularly useful.1-3,11
References
1. Overview of chronic kidney disease-mineral and bone disorder (CKD-MBD) [Internet]. UpToDate. 2018 [cited 2018 Jun 4]. Available from: https://www.uptodate.com/contents/overview-of-chronic-kidney-disease-mineral-and-bone-disorder-ckd-mbd. [ Links ]
2. Kidney International Supplements. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD); 2017. [ Links ]
3. Darryl Quarles L. Bone Disorders in Chronic Kidney Disease. National Kidney Foundation Primer on Kidney Diseases:476–87. [ Links ]
4. Malluche HH, Mawad HW, Monier-Faugere M-C. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011 Jun;26(6):1368–76. [ Links ]
5. Covic A, Voroneanu L, Apetrii M. PTH and/or Bone Histology: Are We Still Waiting for a Verdict From the CKD-MBD Grand Jury? Am J Kidney Dis. 2016 Apr;67(4):535–8. [ Links ]
6. Briot K. Bone and glucocorticoids. Ann Endocrinol. 2018 Jun;79(3):115–8. [ Links ]
7. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney International Supplements; 2009. [ Links ]
8. El-Husseini A, Wang K, Edon AA, Sawaya BP. Parathyroidectomy-A last resort for hyperparathyroidism in dialysis patients. Semin Dial. 2017 Sep;30(5):385–9. [ Links ]
9. Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the Management of Secondary Hyperparathyroidism. Clin J Am Soc Nephrol [Internet]. 2018 Mar 9; Available from: http://dx.doi.org/10.2215/CJN.10390917. [ Links ]
10. Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015 Apr;87(4):846–56. [ Links ]
11. Martin KJ, Floege J, Ketteler M. Bone and Mineral Metabolism in Chronic Kidney Disease. Comprehensive Clinical Nephrology, 5/e.:984–99. [ Links ]
Miguel Bigotte Vieira, MD
Serviço de Nefrologia e Transplantação Renal, Centro Hospitalar
Lisboa Norte, Av. Prof. Egas Moniz, 1649-035 Lisbon, Portugal.
E-mail: mbigottevieira@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Jul 8, 2018
Accepted in revised form: ct 24, 2018














