Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.3 Lisboa set. 2017
ORIGINAL ARTICLE
Echocardiography and cardiovascular risk: The relationship in the renal transplant recipient
Miguel Gonçalves1, Micaela Neto2, Nicole Pestana1, Luís Resende1, Pedro Vieira1, Susana Gomes2, José Durães1, Nuno Rosa1, José Alves Teixeira1, Gil Silva1
1 Department of Nephrology, Hospital Central do Funchal
2 Department of Cardiology, Hospital Central do Funchal
ABSTRACT
Introduction: ardiovascular disease (CVD) is the major cause of death among renal transplant recipients (RTR). It is not known whether echocardiographic abnormalities are useful to identify RTR at high risk of CVD.
Methods: Retrospective review of RTR with functioning and stable graft and an echocardiography performed in the last year. Risk of major adverse cardiac events (MACE) and death using a risk calculator specific for RTR.
Results: Among 107 patients (57.9% males, 50.4±13.9 years), 7-year risk of MACE was >10% in 30.9% of patients and 7-year risk of death >10% in 56.1%. Left ventricular hypertrophy (LVH) was found in 55.1%, diastolic dysfunction in 39.3%, dilated left atrium (LA) in 53.3%, high pulmonary artery systolic pressure (PASP) in 29.0%, valvular calcifications in 22.4% and moderate to severe mitral regurgitation (MR) in 3.7%. Mean Ejection fraction was 68.36±6.87%. Univariate analysis showed an increased risk of MACE and death in patients with LVH, diastolic dysfunction, dilated LA, high PASP, valvular calcifications and MR. Multivariate analysis identified an independent association between the risk of MACE >10% and valvular calcifications and high PASP. Risk of death>10% in multivariate analysis had an independent association with diastolic dysfunction and elevated PASP.
Conclusion: Echocardiographic abnormalities identify RTR at increased risk of MACE and death. Valvular calcifications and high PASP are predictors of MACE whereas diastolic dysfunction and high PASP predict death.
Key words: ardiovascular risk calculator; Diastolic dysfunction; Echocardiography; Pulmonary hypertension; Renal transplant recipient; Valvular calcifications.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with end-stage renal disease (ESRD).1,2 It is becoming clear that mortality in these patients is substantially higher than that predicted from analysis of traditional cardiovascular risk factors, suggesting that multiple cardiac effects of renal disease must be involved.3
These changes are the result of direct uraemic toxicity, cardiovascular calcification and fibrosis, coronary artery disease and hypertension, and have been collectively referred to as uremic cardiomyopathy.1-4 Therefore, while left ventricular hypertrophy (LVH) is the initial manifestation and hallmark of this syndrome, in the more advanced stages, uraemic cardiomyopathy can present with left ventricular (LV) dilatation, along with systolic dysfunction and left atrial (LA) dilatation. Furthermore, these changes confer a poorer prognosis and are an independent predictor of mortality, and the regression of LVH is associated with reduced cardiovascular risk and improved survival.1,5
Haemodialysis is the most common treatment for uraemic cardiomyopathy,3 but it seems to be clear that renal transplantation is a highly effective treatment for CVDs associated with progressive renal disease and confer a significant survival advantage over haemodialysis.2,4 In fact, kidney transplantation compared to dialysis promotes a significant reduction of cardiovascular risk. However, even in the presence of good transplant function, mortality rates due to CVD in renal transplant recipients (RTR) are greater than in the general population.6-8
As previously demonstrated, cardiovascular risk is difficult to assess. The risk-prediction equations defined and validated for the general population underestimate VD risk of kidney transplant recipients because risk factors pertinent to RTR are not included in those equations.9 Transthoracic echocardiography can aid the prediction of cardiovascular events and death in ESRD, but it is not known whether echocardiographic abnormalities are useful to identify RTR with high cardiovascular risk and risk of death.
The aim of the current study was to characterize the metabolic profile, risk of major adverse cardiac events (MACE) and death in a population of RTR, and characterize cardiac function and morphology and determine which echocardiographic abnormalities predict the risk of MACE and death.
SUBJECTS AND METHODS
We collected detailed clinical data from 121 RTR in follow-up, and selected the recipients with a functioning and stable graft for longer than 12 months and a routine echocardiogram performed the previous year in our institution.
Recipient data elements included age and gender, race, primary cause of kidney failure, time from ESRD onset to transplant (dialysis duration), dialysis modality prior to transplant, number of transplants, time functioning graft until time of the analysis, smoking status (current, former or never) and medical history (medication, coronary artery disease, diabetes mellitus, hypertension). Donor data elements included donor (deceased or living). Follow-up data included immunosuppressive regimen, physical examination findings (anthropometric scores, systolic and diastolic blood pressure) and laboratory variables (serum creatinine and lipid values). Race was defined as white, black or other.
Coronary artery disease was defined as the presence of stable angina or history of myocardial infarction (10, 11), Hypertension was defined by systolic blood pressure≥130mmHg, diastolic ≥80mmHg12 or antihypertensive medication use. Diabetes was defined by the use of insulin or oral hypoglycaemic medications or patient history, including type 1 and type 2 diabetes mellitus diagnosed before transplantation and posttransplant diabetes mellitus. Reference values for total cholesterol level, LDL cholesterol level, HDL cholesterol level and hypertriglyceridaemia was based on the final report from the national cholesterol education program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults.13 Body mass index (BMI) was calculated using the formula weight (Kg)/height(m). Classification is according to the cut-off points of the World Health Organization.14
Estimated glomerular filtration rate (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease Study equation, based on serum creatinine level, age, gender, and race.15
MACE was defined as cardiac death, nonfatal myocardial infarction, or coronary revascularization procedure.16
Formula for 7-year MACE and mortality risk calculation for renal transplant recipients16 was used, accessing the Web page Cardiovascular Risk Calculator for Renal Transplant Recipients (http://www.medsci.uu.se/research/renal-medicine/risk-calculator) Variables diabetes mellitus (including post-transplant diabetes mellitus), LDL cholesterol level, smoking status, coronary heart disease, number of transplants, serum creatinine and age were used to calculate 7-year risk of MACE. diabetes mellitus, smoking status, coronary heart disease, total time on renal replace treatment, serum creatinine and age were used to calculate 7-year risk of death.17
All echocardiographic images were stored digitally and analyzed off-line by the same operator, to minimize inter-operator variabilities. The echocardiograms were standard transthoracic echocardiograms performed at the individual sites in accordance with a standard imaging protocol.
The following echocardiographic parameters were ascertained: left ventricular systolic and diastolic function, left ventricular hypertrophy, left atrium volume, valvular calcification, severe mitral regurgitation and pulmonary artery systolic pressure. The cardiac anatomy was assessed by using the M-Mode and two-dimensional (2D) echocardiography.
Measurements of the left ventricle were evaluated by M-Mode tracing of the left ventricle plane and it was considered hypertrophied if septal thickness was superior to 10mm in women and 11mm in men and if posterior wall thickness was superior to 10mm in women and 11mm in men. To quantify the left atrium volume, its border in systole in the apical four-chamber and two-chamber views was traced. Left atrium was considered dilated if its biplane index volume was equal or superior to 34ml/m2.18
The assessment of the left ventricular systolic function was made calculating the left ventricular internal dimension in diastole and systole, using the M-Mode, according to Teichholzs formula.19 An integrated approach to the left ventricular diastolic function was used, recording the mitral inflow velocities by pulsed wave Doppler (peak of early filling [E velocity]; peak of late atrial filling [A velocity]; the E/A ratio; deceleration time [DT] of E velocity) and by recording the pulsed wave tissue Doppler annular diastolic velocities and determining the E/E ratio.20
Doppler echocardiography was used to estimate the pulmonary artery systolic pressure (PASP) and mitral regurgitation. The calculation of PASP was based on measurement of the velocity in the tricuspid regurgitant jet, which reflected the right ventricle to right atrium pressure difference as stated in the Bernoulli equation. Pulmonary hypertension was considered if PASP was superior to 35mmHg.21 Mitral regurgitation was assessed by colour Doppler and continuous wave (CW) Doppler: W Doppler signal strength of the regurgitant flow was compared to the antegrade flow and the regurgitant orifice area (ROA) was calculated. Severe mitral regurgitation was defined by a ROA >= 40mm2 if organic aetiology or >= 20mm2 if functional aetiology.22
Results were expressed as mean values ± SD. Univariate analysis was performed using the chi-square test and the Student T-test for categorical and continuous data, respectively. Variables were used in the logistic regression model to estimate Odds Ratio (OR) for higher rate (>10%) of 7-year MACE and death. All p-values were two-tailed and a P value <.05 was considered significant. All statistical analyses were performed on SPSS v22.0 software for Windows.
RESULTS
Among 121 adult renal transplants recipients followed at our centre, we selected 107 recipients with a functioning and stable graft for longer than 12 months and a routine echocardiography performed in the last year in our institution. The baseline characteristics of the study population are displayed in Table I.
The mean age was 50.4±13.9 years, and the prevalence of males was 57.9%. Prior to transplantation, 90 patients (84.1%) were on haemodialysis; 11 (10.3%) peritoneal dialysis. Six patients (5.6%) received a preemptive kidney transplant. The mean dialysis duration was 49.0±40.8 months (minimum, 0 month; maximum, 189 months). Mean time functioning graft was 115.9±71.8 months (minimum, 13 months; maximum, 370 months). Recipients showed a mean eGFR calculated of 54.2±16.1 mL/min/1.73m2.
The prevalence of coronary artery disease risk factors was as follows: male gender, 62 (57.9%); hypertension, 92 (86.0%); diabetes, 23 (21.5%); lipid abnormalities, 61 (57.0%); cigarette smoking (current or former), 27 (25.2%); and BMI 26.4±4,.; The assessment of the severity of hypertension, performed by the number of hypotensive drugs, was as follows: 1 drug, 25 (23.4%); 2 drugs, 38 (35.5%); 3 drugs 23 (21.5 %); and 4 drugs, 6 (5.6%).
The detailed evaluation of the lipid profile is presented in Table I. In patients analysed, 56 were under statins.
Using the Cardiovascular Risk Calculator for Renal Transplant Recipients17, the 7-year risk of MACE was less than 10% in 74 (69.1%) patients, of 10–20% in 16 (15.0%) patients and over 20% in 17 (15.9%) patients.
The 7-year risk of death was less than 10% in 47 (43.9%) patients, of 10-20% in 31 (29.0%) patients and over 20% in 29 (27.1%) patients.
The transthoracic echocardiography findings are presented in Table 2. According to Teichholzs formula, only one patient had decreased ejection fraction, and among our recipients the mean ejection fraction was 68.4%±6.9%. However the majority of patients had echocardiographic abnormalities. Left ventricular hypertrophy (LVH) was observed in 55.1%, including 49 (45.8%) with mild and 10 (9.3%) with moderate left ventricle thickening. Diastolic dysfunction was present in 39.3% of recipients. In more detail, 10.3% of patients had diastolic dysfunction Grade 1, 28.0% of patients had diastolic dysfunction Grade 2, and 0.9% of patients had diastolic dysfunction Grade 3. Left atrial dilatation (corrected for body surface area) was present in 53.3% of patients, divided into 3 classes: mild dilation 17.8%, moderate dilation 15.0% and severe dilation 20.6%.
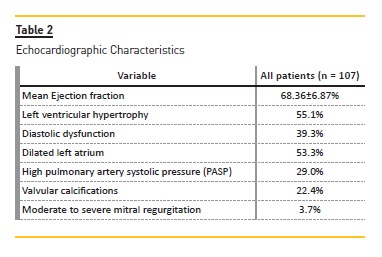
We found an elevated prevalence of high pulmonary artery systolic pressure (PASP) and valvular calcifications. High PASP was present in 28.9% patients, stratified into mild PASP, 25.2%; and moderate PASP, 3.7%. Valvular calcifications were observed in 22.4% of patients. There were 3.7% patients with moderate to severe mitral regurgitation.
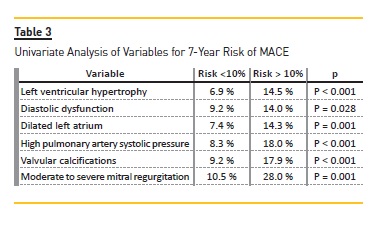
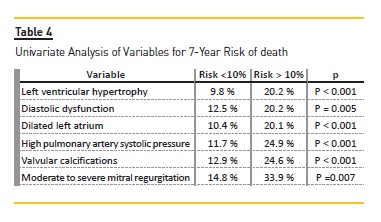
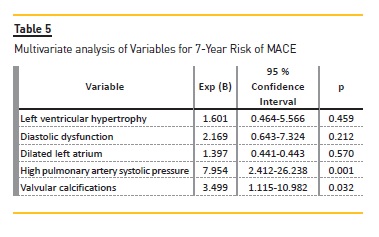
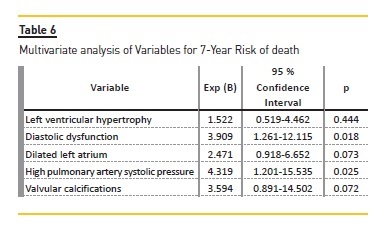
Univariate analysis of variables for 7-year risk of MACE (Table 3) showed that patients with LVH [6.9% vs 14.5% (p<0.001)], diastolic dysfunction [9.2% vs 14.0% (p=0.028)], dilated left atrial [7.4% vs 14.3% (p=0.001)], elevated PASP [8.3% vs 18% (p<0.001)], valvular calcifications [9.2% vs 17.9% (p<0.001)] and moderate to severe mitral regurgitation [10.5% vs 28.0% (p=0.001)] were significant risk factors for the increased (>10%) 7-year risk of MACE. Logistic regression model revealed that valvular calcifications [OR 3.499 (1.115-10.982, P=0.032)] and elevated PASP [OR 7.954 (2.412-26.238, p=0.001)] were independent risk factors to predict a higher risk of MACE (>10%). (Table 5) Regarding the 7-year risk of death (Table 4), patients with LVH [9.8% vs 20.2% (p<0.001)], diastolic dysfunction [12.5% vs 20.2% (p=0.005)], dilated LA [10.4% vs 20.1% (p<0.001)], elevated PASP [11.7% vs 24.9% (p<0.001)], valvular calcifications [12.9% vs 24.6% (p<0.001)] and MR [14.8% vs 33.9% (p=0.007)], were significant risk factors for the increased (>10%) 7-year risk of death. Logistic regression model revealed that diastolic dysfunction [OR 3.909 (1.261-12.115, p=0.018)] and elevated PASP [OR 4.319 (1.201-15.535, p=0.025)] were independent risk factors to predict a higher risk of death (>10%). (Table 6).
DISCUSSION
Clinicians are highly motivated to screen for CVD before transplant, hoping to prevent events early after transplant.23 However, after this initial emphasis, CVD is almost neglected. Nonetheless, the mortality rates due to cardiovascular disease in renal transplant recipients are greater than in the general population.6-8 This is the result of an increase in the prevalence of traditional risk factors and accumulation of several nontraditional risk factors, such as deceased donors,24 longer duration of pre-transplant dialysis,8,25 acute rejection episodes and the use of immunosuppressive drugs.6-8,26
Cardiovascular risk-prediction equations defined and validated for the general population underestimate CVD risk of renal transplant recipients. Therefore, we used a risk calculator specifically tailored for renal transplant recipients.16,17 Our results showed an elevated 7-year risk of MACE and death in our RTR population. According to the prediction calculator,16,17 30.9% and 15.9% of our population had an estimated probability of 7-year MACE over 10% and 20%, respectively. Mortality risk calculation16,17 showed that 56.1% and 27.1% of our population had an estimated probability of 7-year death over 10% and 20%, respectively. As we already presented in another study,9 these results support the concept that RTR have increased risk of cardiovascular complications and death. The application of this calculator individually for each patient allows the clinical risk-stratification.
In fact, due to the complexity of the risk factors, it is difficult to judge the overall cardiovascular risk the patient will incur after kidney transplantation. The application of these calculators can bring benefits enabling more advisable evaluations, offering a way to select patients who benefit from more expensive and invasive exams pre-transplant.
Additionally, it allows the selection of patients who benefit from individualized treatment post-transplant, including the handling of transplantation-specific factors such as immunosuppression, to prevent future events.
The final objective of our study was to characterize morphology and cardiac function by transthoracic echocardiography, and with this identify the abnormalities that may better signal cardiovascular risk. However, our work has some limitations that make it difficult to interpret and extrapolate the results. It is an observational study in a small heterogeneous population. On the other hand, to our knowledge, it is the largest series of echocardiographic evaluations in transplanted patients; we minimized error by having the same operator perform the echocardiographic evaluations , and using a score to stratify RTR based on cardiovascular and death risk, we reduced the bias of population heterogeneity, as the analysis was performed from the risk of MACE and death and not the transplanted population multiple variables.
Several echocardiographic abnormalities were considered. Despite the limited data, we found a great association of our results with the existing literature, especially in the fields of diastolic dysfunction, elevated PASP and valvular calcifications.
Diastolic dysfunction was present in 39.3% of RTR in our population. According to Lodi et al. this change is present in 70% of patients on dialysis.27 Since autopsy studies identified uraemia as an independent determinant of these change27, it is possible that resolution of the uraemic state and euvolaemia status by kidney transplantation could be responsible for less dysfunction index in our population. There is increasing evidence of the association between diastolic dysfunction and increased mortality in chronic kidney disease.28
In our population, the association between the presence of diastolic dysfunction and the high prediction of the 7-year risk of MACE and 7-year risk of death was also clear.There has been increasing interest in studying the association between pulmonary hypertension (PH) and CKD. The exact cause is not proven, and a variety of mechanisms has been proposed to contribute to the development of PH in patients with renal failure.29 Based on echocardiographic studies, the prevalence of PH in ESRD patients maintained on long-term haemodialysis is estimated to be around 26.6–56%.29-35 According to Agarwal et al. echocardiogram timing can influence the results, and low prevalence has been found in measurements in the post early dialysis period. 36,37 In our study group the prevalence of PH was 28.9%. It is lower than the reported range in the literature for patients receiving haemodialysis. Data on the impact of renal transplant on PH are scarce and contradictory. Casas-Aparicio et al.34 assessed 35 patients before and after renal transplant, using echocardiography. They found a significant reduction in pulmonary hypertension in the echocardiography performed 12 months after renal transplantation (48.6% before and 14.3% after transplantation). Another study reported an amelioration of PH after kidney transplantation in 5 patients.35 However, authors argue that this improvement may be due to the optimal volume status of RTR, and the transplant does not reverse pulmonary hypertension, rather correct some of the predictors of PH, such as uraemic toxins.37 In fact, as previously discussed, transplantation appears to confer a reduction in LVH and diastolic dysfunction, which are also predictive of PH36,38. Pabst et al.31 compared the PH prevalence between a dialysis population and a pre-dialysis (CKD 4 and 5) population and found no difference between the two groups. This suggests that the onset of the PH may precede dialysis treatment in many patients, which is not reversible despite renal replacement therapy. Regardless of the mechanism, prolonged elevation in PASP leads to right ventricular dysfunction and consequent morbidity and mortality.30 Several studies have shown that PH is an independent predictor of mortality in patients with ESRD.32,33 As demonstrated in our population, RTR with pulmonary hypertension are at increased risk of MACE. Our results further demonstrate that pulmonary hypertension is an independent factor to predict a higher risk of MACE and death. Owing to the heterogeneity of results, more specific and prospective data are needed to understand this important complication of CKD.
Cardiovascular calcification is a common complication in patients with CKD,39 and is strongly associated with cardiovascular events and mortality.38 Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Working Group guidelines for CKD-MBD40 propose echocardiography detection of valvular calcification and suggest that patients with known valvular calcification must be considered at highest cardiovascular risk. The prevalence of valvular calcification found in our RTR was 22.4%. This result is similar to the 24.4–29% range found by other studies in RTR.39,41 The impact of kidney transplantation on vascular calcification remains poorly understood, and few studies have discussed the matter.
A preliminary study of Moe et al.42 suggested that renal transplantation slows down or arrest coronary artery calcification, whereas in haemodialysis patients still occurs progression of coronary artery calcification The few data are largely consistent with the Moe et al. conclusions.
Indeed, 50–100% of patients on hemodialysis have valvular calcification, approximately two to five times more than RTR.39,41 Existing data should be interpreted with caution, since patients who are transplanted and those who remain on dialysis have significant differences in baseline characteristics.43,44 However, it is tempting to speculate that renal transplantation not only suspends the intense process of vascular calcification, but also improves vascular calcification, as documented by De Lima et al.45 in a prospective study of RTR vascular ultrasound which showed intima-media thickness normalization by transplantation. Valvular and vascular calcifications contribute to cardiovascular mortality risk in dialysis recipients.41 With respect to transplanted patients, our logistic regression model showed that valvular calcifications were an independent risk factor for predicting higher risk of MACE.
As a result of the high prevalence and prognosis of echocardiographic abnormalities, and in accordance with the KDIGO-CKD-MBD Working Group guidelines for CKD-MBD,40 we suggest the performance of echocardiographic evaluations after renal transplantation.
However, we draw attention to the need to develop more extensive and specific consensus in diagnosis and approach of post-transplant cardiovascular disease.
In conclusion, cardiovascular risk calculator specific for RTR can be a useful tool from the time of the pretransplant evaluation, allowing guidance of efforts in the cardiovascular evaluation of the patients with greater risk. Echocardiography is an invaluable and noninvasive tool in the management and prognostic prediction, but its usefulness to identify RTR at increased risk of MACE and death is far from being used to its full capacity. We suggest further studies in this field, and more attention on clinical significance of valvular calcifications, elevated PASP and diastolic dysfunction in RTR. Regardless of the high prevalence of echocardiographic abnormalities, these patients require more aggressive treatment of modifiable cardiovascular risk factors.
References
1. Patel RK, Pennington C, Stevens KK, Taylor A, Gillis K, Rutherford E, et al. Effect of left atrial and ventricular abnormalities on renal transplant recipient outcome-a singlecenter study. Transplant Res. 2014;3(1):20. [ Links ]
2. Josephson CB, Delgado D, Schiff J, Ross H. The effectiveness of renal transplantation as a treatment for recurrent uremic cardiomyopathy. Can J Cardiol. 2008;24(4):315-7. [ Links ]
3. Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M. Uremic cardiomyopathy: an underdiagnosed disease. Congest heart fail. 2013;19(4):E40-5. [ Links ]
4. Huffman C, Wagman G, Fudim M, Zolty R, Vittorio T. Reversible cardiomyopathies-a review. Transplant proc. 2010;42(9):3673-8. [ Links ]
5. Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, et al. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55(6): 1088-96. [ Links ]
6. Marcen R. Cardiovascular risk factors in renal transplantation--current controversies. Nephrol Dial Transplant. 2006;21(3):iii3-8. [ Links ]
7. Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10(2):338-53. [ Links ]
8. Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol . 2000;11(9):1735-43. [ Links ]
9. Goncalves M, Vieira P, Resende L, Duraes J, Rosa N, Teixeira JA, et al. Metabolic profile and cardiovascular risk in a population of renal transplant recipients. Transplant proc. 2015;47(4):985-8. [ Links ]
10. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-67. [ Links ]
11. Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949-3003. [ Links ]
12. National Guideline C. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. 2012. [ Links ]
13. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-421. [ Links ]
14. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [ Links ]
15. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-54. [ Links ]
16. Soveri I, Holme I, Holdaas H, Budde K, Jardine AG, Fellstrom B. A cardiovascular risk calculator for renal transplant recipients. Transplantation. 2012;94(1):57-62. [ Links ]
17. Sciences UUDoM. Cardiovascular Risk Calculator for Renal Transplant Recipients 2015 [updated July 14, 2017; cited 2015. Available from: http://www.medsci.uu.se/research/renal-medicine/risk-calculator. [ Links ]
18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. [ Links ]
19. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37(1):7-11. [ Links ]
20. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165-93. [ Links ]
21. Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, et al. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol . 1987;59(6):662-8. [ Links ]
22. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with twodimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777-802. [ Links ]
23. Hart A, Weir MR, Kasiske BL. Cardiovascular risk assessment in kidney transplantation. Kidney Int. 2015;87(3):527-34. [ Links ]
24. Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol. 2002;13(4):1084-90. [ Links ]
25. Abbott KC, Bucci JR, Cruess D, Taylor AJ, Agodoa LY. Graft loss and acute coronary syndromes after renal transplantation in the United States. J Am Soc Nephrol . 2002;13(10):2560-9. [ Links ]
26. Fellstrom B. Risk factors for and management of post-transplantation cardiovascular disease. BioDrugs . 2001;15(4):261-78. [ Links ]
27. Losi MA, Memoli B, Contaldi C, Barbati G, Del Prete M, Betocchi S, et al. Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant . 2010;25(6):1950-4. [ Links ]
28. Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol . 2007;99(3):393-8. [ Links ]
29. Bozbas SS, Kanyilmaz S, Akcay S, Bozbas H, Altin C, Karacaglar E, et al. Renal transplant improves pulmonary hypertension in patients with end stage renal disease. Multidiscip Respir Med . 2011;6(3):155-60. [ Links ]
30. Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. 2013;84(4):682-92. [ Links ]
31. Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohe C, Nickenig G, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PloS one. 2012;7(4):e35310. [ Links ]
32. Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28(6):990-7. [ Links ]
33. Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75(9):969-75. [ Links ]
34. Casas-Aparicio G, Castillo-Martinez L, Orea-Tejeda A, Abasta-Jimenez M, Keirns-Davies C, Rebollar-Gonzalez V. The effect of successful kidney transplantation on ventricular dysfunction and pulmonary hypertension. Transplant Proc. 2010;42(9):3524-8. [ Links ]
35. Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123(5):1577-82. [ Links ]
36. Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant. 2012;27(10):3908-14. [ Links ]
37. Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37(3):281-90. [ Links ]
38. Cianciolo G, Capelli I, Angelini ML, Valentini C, Baraldi O, Scolari MP, et al. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol . 2014;39(5): 418-26. [ Links ]
39. Leskinen Y, Paana T, Saha H, Groundstroem K, Lehtimaki T, Kilpinen S, et al. Valvular calcification and its relationship to atherosclerosis in chronic kidney disease. J Heart Valve Dis. 2009;18(4):429-38. [ Links ]
40. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009(113):S1-130. [ Links ]
41. Kraus MA, Kalra PA, Hunter J, Menoyo J, Stankus N. The prevalence of vascular calcification in patients with end-stage renal disease on hemodialysis: a cross-sectional observational study. Ther Adv Chronic Dis. 2015;6(3):84-96. [ Links ]
42. Moe SM, ONeill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA. Natural history of vascular calcification in dialysis and transplant patients.Nephrol Dial Transplant. 2004;19(9):2387-93. [ Links ]
43. Moe SM, ONeill KD, Fineberg N, Persohn S, Ahmed S, Garrett P, et al. Assessment of vascular calcification in ESRD patients using spiral CT.Nephrol Dial Transplant . 2003;18(6):1152-8. [ Links ]
44. Stompor T, Pasowicz M, Sulowicz W, Dembinska-Kiec A, Tracz W. Trends in coronary artery calcification in peritoneal dialysis and transplant patients. Nephrol Dial Transplant. 2004;19(12):3205-6; author reply 6. [ Links ]
45. De Lima JJ, Vieira ML, Viviani LF, Medeiros CJ, Ianhez LE, Kopel L, et al. Long-term impact of renal transplantation on carotid artery properties and on ventricular hypertrophy in end-stage renal failure patients. Nephrol Dial Transplant. 2002;17(4):645-51. [ Links ]
Miguel Gonçalves
Department of Nephrology
Hospital Central do Funchal
Avenida Luís de Camões nº 57, 9004-514, Funchal, Portugal
E-mail: amvg.leugim@gmail.com
+351 291 705600, Fax +351 291 705653
Disclosure of potential conflicts of interest: none declared
Authors Contributions: MG, MN and LR made substantial contributions to the study concept and design, analysis and interpretation of data, and were involved in drafting the manuscript and revising it critically for important intellectual content. NP, PV and SG were also involved in drafting the manuscript and revising it critically for intellectual content. JD, NR, JAT and GS were involved in the critical revision of the manuscript.
Received for publication: Aug 15, 2017
Accepted in revised form: Sep 21, 2017














