Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.2 Lisboa jun. 2016
ORIGINAL ARTICLE
Mineral metabolism and inflammation: factors related to left ventricular hypertrophy in patients with diabetic nephropathy
Teresa Jerónimo1, André Fragoso1, Filipa Mendes1, Ana Paula Silva1,2, Ana Pimentel1, Nelson Tavares3, Ana Camacho3, Pedro Leão Neves1,2
1 Nephrology, Centro Hospitalar do Algarve, Faro, Portugal
2 Department of Biomedical Sciences and Medicine, University of Algarve
3 Cardiology, Centro Hospitalar do Algarve, Faro, Portugal
ABSTRACT
Left ventricular hypertrophy (LVH) is an important risk factor for cardiovascular disease in patients with diabetic nephropathy (DN) and is an independent predictor of mortality in patients with chronic kidney disease (CKD).
The aim of this study was to evaluate the association of LVH with mineral metabolism and inflammation in a population of patients with DN. In an observational study were included 119 type 2 diabetic patients with CKD stages 3 and 4. The population was divided into two groups, according to the presence of LVH: group 1 (G-1) with LVH (left ventricular mass index (LVMI) > 125 g/m2 in male patients and LVMI > 110 g/m2 in female patients) and group 2 (G-2) without LVH (LVMI ≤ 125 g/m2 in male patients and LVMI ≤ 110 g/m2 in female patients). The patient characteristics of each group were compared regarding several biological and laboratory parameters.
Patients with LVH displayed lower values of estimated glomerular filtration rate (eGFR) (p = 0.0001) and albumin (p = 0.046), and higher levels of phosphorus (p = 0.0001), intact parathyroid hormone (iPTH) (p = 0.0001), insulin resistance (HOMA-IR) (p = 0.0001) and interleukin-6 (IL-6) (p = 0.0001), compared with patients without LVH. In a logistic regression model, phosphorus (odd ratio (OR) = 1.825 (1.075-4.414), p = 0.038), iPTH (OR = 1.991 (1.098-3.000), p = 0.004) and IL-6 (OR = 3.538 (1.863-6.719), p = 0.0001) were independently related to LVH. In a multiple linear regression model, phosphorus (r = 0.602, p = 0.038), iPTH (r = 1.009, p = 0.044) and IL-6 (r = 1.264, p = 0.0001) were positively related to LVMI. Phosphorus, PTH and IL-6 were related to LVH in our diabetic population with CKD stages 3 and 4.
Key-Words: Chronic kidney disease; diabetic nephropathy; interleukin-6; left ventricular hypertrophy; parathormone; phosphorus.
INTRODUCTION
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease (ESRD)1 and its mortality is mainly due to cardiovascular disease (CVD) 2.
Compared with general population, a large proportion of patients with chronic kidney disease (CKD) die from CVD3. Left ventricular hypertrophy (LVH) is an independent risk factor for CVD4,5.
Previous reports have demonstrated that non-traditional risk factors, such as parathyroid hormone (PTH) and phosphorus are involved in the pathogenesis of a number of cardiovascular (CV) abnormalities in CKD patients, including LVH6,7.
Recently, the impact of inflammation on LVH in CKD patients have gained attention in nephrology8. The aim of our study was to evaluate the association of LVH with mineral metabolism and inflammation in a population of type 2 diabetic patients with nephropathy.
MATERIALS AND METHODS
Study population
In a cross-sectional study were included 119 type 2 diabetic patients, recruited between December 2007 and May 2013 with diagnosis of CKD in a stable clinical condition attending our outpatient clinic. The classification of diabetes was based on the guidelines from the American Diabetes Association9. The CKD stages were defined by the estimated glomerular filtration rate (eGFR) calculated with the modification of diet in renal disease (MDRD) formula at the time of assessment10. Exclusion criteria were previous CVD – defined as a history of one or more of the following: non-fatal myocardial infarction, angina pectoris (stable or unstable), stroke or transient ischaemic attacks, peripheral vascular disease or congestive heart failure; uncontrolled hypertension (BP ≥ 140/90mmHg), urine albumin-to-creatinine ratio (UACR) > 500, eGFR ≤ 15mL/min or > 80mL/min, type 1 diabetes, non-diabetic renal disease, neoplastic or infectious diseases. The study was approved by the local Ethnics Committee, and written informed consent was obtained from each participant.
The study was conducted according to the principles of the Declaration of Helsinki.
Follow-up
Follow-up of patients was conducted 2-3 times a year during in-person visits on nephrology consultation.
Patients with more severe conditions returned approximately every 3 months, while the other patients returned approximately every 6 months. No patient was lost to follow-up.
Blood measurements
Several laboratory parameters were determined using a standard methodology in routine blood samples drawn after an overnight fast. The serum levels of calcium and phosphorus were measured using the ARCHITECT c Systems and the AEROSET System (Abbott Diagnostics Division, Abbott Laboratories Abbott Park, IL, USA). Interleukin-6 (IL-6) levels were measured using a sandwich enzyme-linked immunoassay (ELISA) kit (eBioscience, San Diego, California, USA). Haemoglobin (Hb) and intact PTH (iPTH) levels were measured using spectrophotometry technique and electrochemiluminescent immunoassay (ECLIA), respectively. The iPTH concentrations were measured on an Immulite 2000 Intact PTH assay (Cat. # L2KPP2, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA).
The degree of insulin resistance (IR) was estimated using homeostatic model assessment (HOMA)-IR described by Matthews et al. 11.
Serum creatinine was measured by enzymatic method, using the ARCHITECT device (Abbott Diagnostics Division, Abbott Laboratories Abbott Park, IL, USA).
Glomerular filtration rate was estimated using a formula derived by MDRD study group10.
Echocardiography
Transthoracic echocardiography was performed using General Electrical Medical Systems echograph, model Vivid 7 with a probe (GE Healthcare, Wisconsin, USA). Left ventricular mass index (LVMI) was calculated by applying the regression equation from the Penn convention12.
Definitions
Left ventricular hypertrophy was defined as LVMI > 125 g/m2 in male patients and LVMI > 110 g/m2 in female patients13. Subjects were classified into two groups accordingly to the presence of LVH: group 1 (G-1) with LVH and group 2 (G-2) without LVH.
Statistical analyses
Analyses were performed by using descriptive statistics, and for comparisons between groups the students t-test and the chi-squared test were used. The factors associated to LVH and their odds ratio (OR) were calculated using multivariate logistic regression. We also used a multiple linear regression model to evaluate the factors that influenced the LVMI. A p-value of < 0.05 was considered significant. Statistical analysis was performed with SPSS 17.0 for Windows.
RESULTS
The clinical characteristics of the population (N = 119) are provided in Table I. The mean age was 62.8 ± 12.43 years, 52.7% were male and 47.3% were female patients. Fifty-five patients had LVH.
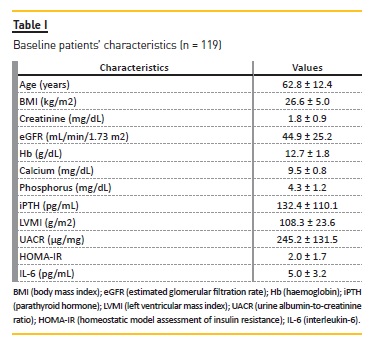
Table II displays a comparison of patients characteristics between G-1 (patients with LVH) and G-2 (patients without LVH). Patients with LVH were older, had lower values of eGFR (p = 0.0001) and albumin (p = 0.046), and higher levels of phosphorus (p = 0.0001), iPTH (p = 0.0001), HOMA-IR (p = 0.0001) and IL-6 (p = 0.0001), comparedwith patients without LVH.
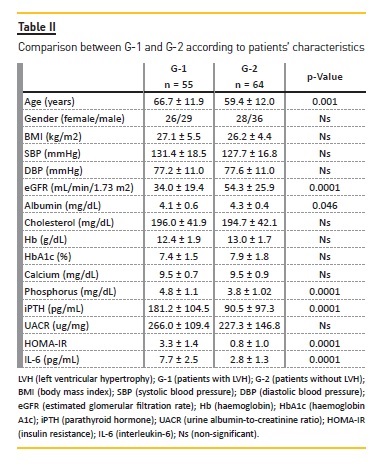
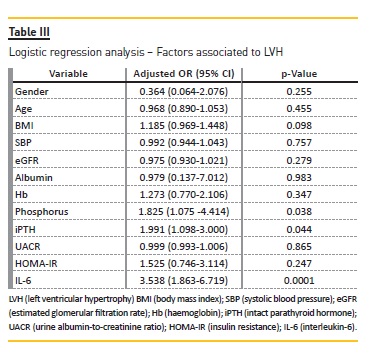
In a logistic regression model, LVH was used as the dependent variable. Gender, age, body mass index (BMI), systolic blood pressure (SBP), eGFR, albumin, Hb, phosphorus, iPTH, UACR, HOMA-IR and IL-6 (Table III) were considered independent variables. In this model, phosphorus (odds ratio (OR) = 1.825 (1.075-4.414), p = 0.038), iPTH (OR = 1.991 (1.098-3.000), p = 0.004) and IL-6 (OR = 3.538 (1.863-6.719), p = 0.0001) were independently related to LVH.
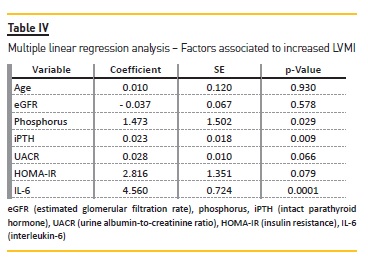
To evaluate possible causes of increased LVMI, a multiple linear regression model adjusted for age, eGFR, phosphorus, iPTH, UACR, HOMA-IR and IL-6 (Table IV) were used. In this model, phosphorus (r = 0.602, p = 0.038), iPTH (r = 1.009, p = 0.044) and IL-6 (r = 1.264, p = 0.0001) were positively related to LVMI.
DISCUSSION
Patients with CKD have a high prevalence of LVH, ranging from 36% to 74% in different studies and its prevalence increases as renal function declines14-16.
Hypertension, hypervolaemia, and anaemia have been identified as major determinants of LVH in a population with CKD17. Other factors, such as inappropriate activation of the renin-angiotensin-aldosterone system, oxidative stress and inflammation, may also play a role in left ventricular growth in ESRD18-20.
In this study, we found that iPTH, phosphorus and IL-6 were independently associated with LVH in type 2 diabetic patients with CKD stages 3 and 4. These findings suggest that disturbances of mineral metabolism and inflammation are linked to LVH in patients with DN.
In the past years, there has been compelling evidence that the cardiovascular system is a major target of PTH action, suggesting that its chronic elevation in ESRD patients adversely affects myocardial metabolism and function21. A recent study suggests that PTH retains substantial independent predictive value for major CV events and for all-cause mortality22. Left ventricular hypertrophy in uraemic patients is not only characterized by an increased myocardial fibre mass but also by myocardial interstitial fibrosis23 and it has been observed that elevated PTH levels in ESRD cause irreversible interstitial fibrosis with collagen deposition24. In vitro studies have shown that PTH appears to have chronotropic, inotropic, as well as hypertrophic effects on cardiomyocytes25. The mechanisms by which PTH induces LVH have not been completely elucidated. Studies have shown increased cytosolic calcium and/or protein kinase C activation and expression of cardiac proto-oncogene may be enhanced, which in turn may lead to altered expression of several genes involved on cardiac structure and action and ultimately stimulate the translation of contractile and noncontractile cardiac muscle proteins leading to LVH26.
Other mechanisms have also been reported to explain the association of secondary hyperparathyroidism and LVH: increased IR and pancreatic ß-cell dysfunction, predisposing to the metabolic syndrome and diabetes; activation of renin-angiotensin system, increasing blood pressure and leading to myocardial cells apoptosis and fibrosis; stimulation of systemic and vascular inflammation and calcification, augmenting atherogenesis27.
Supporting a causal CV role, PTH receptors have been discovered in the heart and vasculature, and surgical parathyroidectomy for primary hyperparathyroidism and renal transplantation for secondary hyperparathyroidism have been reported to reduce this risk of CVD28-30. Following parathyroidectomy in animals with chronic renal failure, a reduction of collagen deposition in the myocardium is consistently observed26. Interactions between PTH levels and cardiac abnormalities specifically related to LVH and left ventricular diastolic dysfunction were shown in patients with primary hyperparathyroidism as well6,31,32. Such abnormalities were independent of plasma calcium levels and hypertension and also regressed following parathyroid mass reduction6,31-33.
Hyperphosphatemia is a cardiovascular risk factor in patients with chronic kidney disease; Dingra et al. demonstrated that serum phosphorus in patients with CKD stages 1 and 2, free of heart failure and ischaemic heart disease, were positively associated with increased LVMI and LVH34. Several attempts have been made to determine the pathophysiology of the relationship between phosphorus and myocardial damage. Recent studies reported severe hyperparathyroidism or hyperphosphatemia and elevated levels of fibroblast growth factor-23 (FGF-23) in dialysis patients35, that were significantly associated with increased LVMI and the probability of developing LVH36,37. It is unclear whether FGF-23 is a mere marker, or a potential mechanism of LVH in patients with CKD.
The consequences of inflammation, a non-traditional risk factor, have gained attention in nephrology. Attention has been attracted to IL-6 with regard to myocardial dysfunction as increased levels are strongly prognostic of mortality38. Increased production of pro-inflammatory cytokines, as IL-6, from microinflammation activation of the sympathetic nervous system in CKD patients, has also been implicated in LVH39,40. Experimental studies have shown IL-6 overexpression in diabetic kidneys, which is associated with glomerular basal membrane thickening in type 2 diabetes patients and mesangial expansion in kidney biopsies of diabetic patients41, and its levels are higher in patients with overt proteinuria compared to microalbuminuria or normoalbuminuria42.
Meléndez et al. found that IL-6 induced significant concentric LVH43 and this finding is consistent with the previous reports by Hirota44
There are several limitations in the current study, such as the relatively small sample size and, consequently, the limited statistical power of the tests applied. Prospective studies with larger sample size and robust statistical analyses are required in order to confirm these associations.
CONCLUSION
In a population of type 2 diabetic patients with CKD stages 3 and 4, phosphorus, PTH and IL-6 were independently related to LVH.
Further studies are warranted to confirm whether a decrease in levels of phosphorus, PTH and IL-6 would reduce the LVH and consequently the CV risk in diabetic type 2 patients with nephropathy.
References
1. U.S. Renal Data System, USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD, 2003. [ Links ]
2. International Diabetes Federation – Diabetes Atlas. Third Edition, Brussels: International Diabetes Federation, 2006. [ Links ]
3. Vanholder R, Massy Z, Argiles A,
4. Cecil MP, Fajman WA, Ziffer JA. The heart in hypertension. N Engl J Med 1993;328(3):212–213. [ Links ]
5. Bombelli M, Facchetti R, Carugo S, et al. Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens 2009;27(12):2458-2464. [ Links ]
6. Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J 2003;24(22):2054–2060. [ Links ]
7. Yamamoto KT, Robinson-Cohen C, Oliveira MC, et al. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int 2013;83(4):707-714. [ Links ]
8. Poulikakos D, Ross L, Recio-Mayoral A, et al. Left ventricular hypertrophy and endothelial dysfunction in chronic kidney disease. Eur Heart J Cardiovasc Imaging 2014;15(1):56-61. [ Links ]
9. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33(1):62-69. [ Links ]
10. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new predition equation. Modification of Diet in Renal Disease Study Group. Annals Int Med 1999;130(6):461-470. [ Links ]
11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412-419. [ Links ]
12. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55(4):613-618. [ Links ]
13. Mancia G, De Backer G, Dominiczak A,
14. Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999;34(1):125-134. [ Links ]
15. Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 2005;46(2):320-327. [ Links ]
16. Nardi E, Palermo A, Mule G, Cusimano P, Cotton S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertensi 2009; 27(3):633-641. [ Links ]
17. Neves PL, Silva AP, Bernardo I. Elderly patients in chronic hemodialysis: risk factors for left ventricular hypertrophy. Am J Kidney Dis 1997; 30(2):224-228. [ Links ]
18. Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS. Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol 1994;4(7):1486-1490. [ Links ]
19. Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995;47(1):186-192. [ Links ]
20. Cerasola G, Nardi E, Palermo A, Mulè G, Cottone S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol 2011;24(1):1–10. [ Links ]
21. Massry SG, Smogorzewski M. The heart in uremia. Semin Nephrol 1996;16(3):214-221. [ Links ]
22. Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am Heart Journal 2011;162(2):331-339. [ Links ]
23. Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant 1990;5(1):39-44. [ Links ]
24. Amann K, Ritz E, Wiest G, et al. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol 1994;4(10):1814-1819. [ Links ]
25. Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest 1981;67(4):1215–1227. [ Links ]
26. Mall G, Rambausek M, Neumeister A, Kollmar S, Vetterlein F, Ritz E. Myocardial interstitial fibrosis in experimental uremia – implications for cardiac compliance. Kidney Int 1988;33(4):804-811. [ Links ]
27. Lee JH, OKeefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008;52(24):1949-1956. [ Links ]
28. Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease–a review. Eur Heart J 2004;25(20):1776-1787. [ Links ]
29. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341(23):1725-1730. [ Links ]
30. Garcia de la Torre N, Wass JA, Turner HE. Parathyroid adenomas and cardiovascular risk. Endocr Relat Cancer 2003;10(2):309-322. [ Links ]
31. Nappi S, Saha H, Virtanen V, et al. Left ventricular structure and function in primary hyperparathyroidism before and after parathyroidectomy. Cardiology 2000;93(4):229-233. [ Links ]
32. Andersson P, Rydberg E and Willenheimer R. Primary hyperparathyroidism and heart disease – a review. Eur Heart J 2004;25(20):1776-1787. [ Links ]
33. Dominiczak AF, Lyall F, Morton JJ, et al. Blood pressure, left ventricular mass and intracellular calcium in primary hyperparathyroidism. Clin Sci (Lond) 1990;78(2):127-132. [ Links ]
34. Dhingra R, Gona P, Benjamin EJ, et al. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur Heart J 2010;12(8):812-818. [ Links ]
35. Komaba M, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int 2010;77(4):292-298. [ Links ]
36. Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009;119(19):2545-2552. [ Links ]
37. Kirkpantur A, Balci M, Gurbuz OA, Baris Afsar, Basol Canbakan, Ramazan Akdemir, Mehmet Deniz Ayli. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant 2011;26:1346-1354. [ Links ]
38. Maeda K, Tsutamoto T, Wada A, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000;36(5):1587-1593. [ Links ]
39. Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens 2008; 17(2):162-167. [ Links ]
40. Ritz E. Left ventricular hypertrophy in renal disease: Beyond preload and afterload. Kidney Int 2009; 75(8):771–773. [ Links ]
41. Dalla Vestra M, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol 2005;16 Suppl 1:s78–s82. [ Links ]
42. Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH. Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 2003;46(10):1402–1407. [ Links ]
43. Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010;56(2):225–231. [ Links ]
44. Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signaltransducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 1995;92(11):4862– 4866. [ Links ]
45. Tsuruda T, Jougasaki M, Boerrigter G, et al. Cardiotrophin-1 stimulation of cardiac fibroblast growth: roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ Res 2002;90(2):128 –134. [ Links ]
46. Sano M, Fukuda K, Kodama H, et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem 2000;275(38):29717–29723. [ Links ]
Teresa Jerónimo
Department of Nephrology, Centro Hospitalar do Algarve – Unidade de Faro
Rua Leão Penedo, Faro, Portugal.
E-mail: teresa_jerónimo@hotmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Jun 15, 2016
Accepted in revised form: Feb 16, 2016














