BACKGROUND
Surgical procedures involve simultaneous administration of several drugs and other substances, such as dyes, disinfectants and contact with latex which may induce hypersensitivity reactions (HR).
The incidence of such events is difficult to estimate due to the heterogeneity of the studies and the variety of medications used worldwide. The incidence of perioperative HR is thought to vary between 1:353 and 1:18,600 procedures, with antibiotics and neuromuscular blocking agents (NMBA) being the most commonly implicated drugs 1. The incidence of HR to blue dyes is increasing due to its extensive use with incidence being reported as high as 1:300 procedures 2.
We present a patient, undergoing unilateral mastectomy, who developed hypersensitivity reaction (HR) to Blue Patent Dye (PBD) injection for sentinel node mapping and describe the allergological work-up performed.
CASE PRESENTATION
The patient was a woman in her forties diagnosed with invasive ductal carcinoma of the left breast under chronic treatment with tamoxifen. Past medical history of depression, medicated with venlafaxine, and uneventful spine surgery under general anesthesia. No history of atopy or allergic disorder, such as food, drug, or latex allergy.
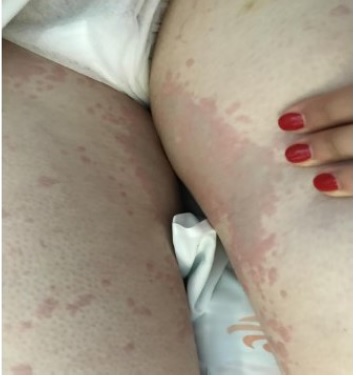
The patient underwent unilateral mastectomy with sentinel lymph node mapping under general anesthesia. After antibiotic prophylaxis with 2 g of cefazolin, anesthesia induction was achieved with 0.2 mg of fentanyl, 140 mg of propofol, 40 mg of rocuronium, 40 mg of lidocaine, and 4 mg of dexamethasone. Then, patent blue dye was administered. About 30 minutes later, the patient developed a violaceous maculopapular rash on the face, trunk, and limbs (Figure 1) and mild laryngeal edema without respiratory or hemodynamic compromise, according to information reported by anesthetists. She was treated with 300 mg of intravenous hydrocortisone and 2 mg of intravenous clemastine with symptoms resolution occurring in 1 hour. No tryptase assay was performed. Due to the clinical and hemodynamic stability, it was decided to proceed with the surgery without further complications and the patient was referred 9 months later to our Allergy Department for study.
The investigation included the patient baseline sérum tryptase (3,2 ng/mL), skin prick tests (SPT), intradermal tests (IDT), basophil activation test (BAT), and drug provocation test (DPT).
We performed SPT with cefazolin, fentanyl, propofol, rocuronium, lidocaine, dexamethasone, patente blue, and methylene blue (the latter as a possible alternative dye), latex and chlorhexidine. SPT with patente blue (Bleu Patenté V sodique® 25mg/mL, Guerbet®) was positive (5 mm wheal with surrounding erythema) in the patient and negative in 3 healthy female volunteers without drug allergy reported. The remaining SPT were all negative, including methylene blue (5mg/ml). IDT with the same substances, including methylene blue (1:100), tested negative. IDT with patent blue was not performed. SPT and IDT were performed according to non-irritating concentrations described in the literature (1) (Table 1). BAT was negative for patente blue and methylene blue. Hypersensitivity reaction to cefazolin, lidocaine, and dexamethasone was excluded based on negative DPT. Rocuronium allergy was also excluded based on negative SPT and IDT. One month after the reaction, the patient underwent a gynecologic surgery where fentanyl and propofol were administered without complications, which allowed us to exclude allergies to these drugs.
Table 1 Concentrations and results of SPT and IDT
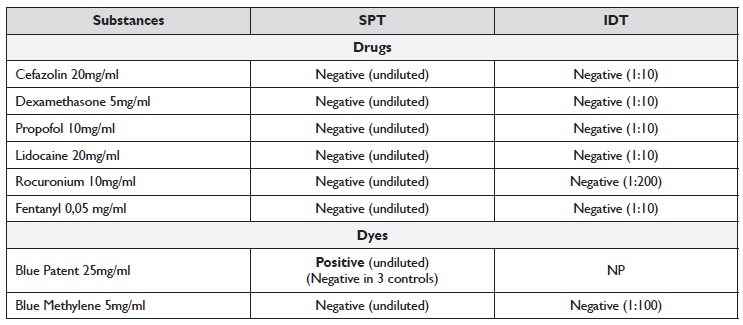
IDT - intradermal tests; NP - not performed; SPT - skin prick tests
Despite the patient claiming that she had never been previously exposed to dyes, according to the study, patente blue was considered the culprit. The patient received written information to avoid patent blue dye and to use methylene blue dye as an alternative, if necessary.
DISCUSSION
This case illustrates a hypersensitivity reaction to PBD during a mastectomy with sentinel lymph node mapping.
The first symptoms usually start 5 to 25 minutes after the injection, with some authors reporting more than 60 minutes 3-5. The most severe reactions tend to happen sooner after dye injection. The blue-colored urticaria is more common in late reactions with the appearance of lesions in other parts of the body, which is probably related to previous exposure to the allergen 4-7. In our patient, the reaction appeared 30 minutes after the injection of dye and involved large areas of the skin and the larynx, although mildly in the latter. We considered it to be an anaphylactic reaction, according to the recently published criteria 8. The same diagnosis was not assumed by the surgical medical team, given that neither epinephrine was administered nor tryptase was measured. It is suggested that reactions to blue dyes are IgE mediated 4,6,7 and so prior exposure and sensitization to the causing agent are needed to develop such reactions.
Frequently these patients appear to react at their first exposure, as in our patient. However, it is possible that a first exposure could previously have occurred due to the widespread use of blue dyes in drugs, food, and objects of everyday life 4-7. In our investigation, an IgE-mediated reaction is confirmed by positive SPT to PBD.
Studies of the clinical usefulness of BAT on PBD allergy have shown contradictory results, with some reporting positive results 9,10 when tested 1 to 92 months after anaphylaxis and other reporting negative ones 7 when performed 49 months later. In our report, BAT performed 9 months after the reaction was negative, so we considered it of limited usefulness.
Regarding alternative dyes, Methylene Blue (MB) and Isosulfan Blue (IB) are the most often used. PBD and IB are chemically closely related, both belonging to the Patent Blue family of dyes. Methylene Blue, with a diferente chemical structure, has been proposed as a safer alternative 4, although cross-reactivity has already been published 7. In our report, SPT and IDT to MB were negative, so we considered it an alternative, although the patient did not need to use another dye in the meantime.
CONCLUSIONS
During an intraoperative HR, in addition to drugs, other agents such as dyes should also be suspected. The allergological work-up is essential to identify not only the culprit drug as well as possible alternatives. Methylene Blue may be an alternative option but it should be properly investigated in an allergy department before use.