INTRODUCTION
Patients and members of the public are getting involved as valuable members in innovative health solutions. The National Institute for Health Research (NHS) in the United States 1, Canada’s Strategy for Patient-Oriented Research 2, and European Lung Foundation (ELF) 3 are examples of successful establishment of Patient and Public Involvement (PPI) in health research. In Portugal, the collaborative network ConectAR - patient and public engagement to advance respiratory disease and digital health research, is one of the most recent examples of initiatives to actively involve patients and carers as co-researchers in patient-oriented research 4,5. PPI in research is focused on the engagement of patients/carers in every stage of the research cycle: identifying and prioritizing, commissioning, designing, managing, undertaking, disseminating, implementing, and evaluating impact 6. Patients contribute with a different and complementary perspective to research, by adding personal knowledge and experience, and ensuring research priorities are aligned with their needs 6. PPI in health research is thus believed to improve its quality, relevance and ethics and ultimately improve patients’ outcomes. Along with the acknowledgment of its importance, many guidance documents, frameworks and reviews have been developed on patients’ involvement in health research 7.
Challenges of PPI have been reported in terms of patient selection, time, training, governance and power- sharing 8-13. The inconsistent level of education and research experience is one of the main causes of disappointment, frustration and powerlessness. Training and support covering topics such as the research cycle, an overview of different research methods and research terminology are recommended to help patients/carers understand the value of their perspective, recognise the skills and experience they bring from other areas of life, and understand where they fit within the research team 6,14. Training may take many formats, for example, group sessions, high-quality written materials, attending conferences, networking and shared learning with peers, online activities and university or college courses. In Portugal, patients’ associations, such as Respira 15 and Associação Portuguesa de Asmáticos 16, play an importante role in supporting patients with chronic respiratory diseases and provide written articles and leaflets on specific disease topics, but to our knowledge do not offer consistent training to empower patients in their health decisions, or to be involved in research or politics. Likewise, the Sociedade Portuguesa de Alergologia e Imunologia Clínica (SPAIC); the Sociedade Portuguesa de Pneumologia (SPP) and Grupo de Estudos de Doenças Respiratórias (GRESP) are committed to the medical and scientific training of their members by offering conferences, courses, webinars, etc, however, these initiatives are commonly not available nor adapted for the patients and public. To engage patients/carers in health research, aiming for the improvement of quality, relevance and impact of research, it is essential to provide high-quality training tailored to patients’/carers’ needs and preferences and grounded on attractive and easily disseminated formats.
OBJECTIVES
Our main goal is to develop and deliver a high-quality and comprehensive online educational programme for adult patients with asthma and their carers, based on patients’/carers’ needs and preferences. Specifically in this project, we aim to:
1) design an interactive online educational programme in co-creation with patients and carers;
2) assess the feasibility and acceptability of the programme;
3) assess the impact of the programme on participants’ knowledge, and self-efficacy in asthma self-management and interest to be involved in research.
METHODS AND ANALYSIS
This self-learning programme will target adult patients with asthma and their carers. It is meant to be delivered online, free of charge and each person may complete it at his/her own pace.
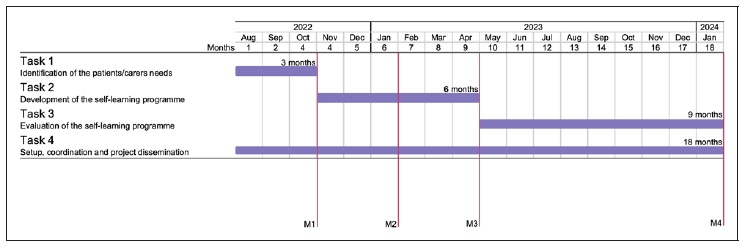
The project includes four tasks: the identification of patients’ and carers’ needs (Task 1); design and delivery of the self-learning programme based on patients’/carers’ preferences (Task 2); evaluation of the feasibility, acceptability and impact of the self-learning programme (Task 3); and the management and dissemination of the project (Task 4). Patients and carers will be actively engaged in all stages of this project as co-researchers. The tasks, described in detail in the following section, are scheduled to be developed in eighteen months (Figure 1).
Tasks
Task 1. Identification of patients’/carers’ needs This task aims to identify and organise the most importante topics on asthma and health research, based on the educational needs of adult patients/carers with asthma and the expertise of asthma specialists. We will use the Delphi method 17 to reach a consensus on the importance of the topics to be addressed (Task 1.1). Then we will organise the most important topics into modules (Task 1.2). This task will take 3 months.
T1.1 Reaching consensus on the educational topics
Adult patients with asthma and carers from the ConectAR network as well as physicians and researchers linked to our research team will be invited to participate in online anonymous web surveys. Multiple interactions (response and feedback) will take place until a consensus on the most important topics to be addressed in an educational programme is reached (defined by a concordance of at least 80% for those classified as “very important”/“important”).
Round 1: A list of topics built by researchers and patients to be addressed in an educational programme will be presented to two independent panels: 30 patients with asthma/carers and 30 asthma specialist physicians and researchers. Both panels will be asked to rank each topic as “very important”/ “important”/ “less important”/ “non-important” and to suggest additional topics. For each topic, the degree of importance and the concordance between panels will be analysed and summarised. A common list of topics, including those added by patients/carers and experts, will be created.
Round 2: The list created in round 1 will be presented and ranked for importance by the same two panels of patients/carers and experts. Each participant will be independently asked to agree/disagree and make further comments. From the ranking being observed in round 1, the research team believes two rounds will be enough to obtain consensus. Nevertheless, further Delphi iterations repeating the process described in round 2, may be needed.
T1.2 Organising the topics in modules
Based on the consensus from T1.1, the most importante topics (those classified as “very important”/ “important”) will be grouped into three educational modules.
Less important topics (consensually classified as “less important” or “non-important”) will be dropped from this initial programme. For each module, the learning objectives will be determined, and the sources of information for content production will be identified among the most recent, high-quality sources of knowledge. This will be delivered to Task 2.
At the end of this task, we expect to have a consensual list of the most important topics to be addressed in the educational programme (Milestone 1, Figure 1). This list will be identified based on the educational needs of adult patients/carers with asthma and the expertise of asthma specialists and will be organised into modules with learning objectives and sources of information established for each module.
Task 2. Development of the self-learning programme This task aims to develop a high-quality, free-ofcharge, online self-learning programme for adult patients with asthma and their carers. The programme will be designed upon reliable sources of information and based on patients’/carers’ preferences (T2.1) and inputs.
Then it will be implemented on a web platform (T2.2) and its usability will be tested (T2.3). This task will take 6 months.
T2.1 Designing the self-learning programme
The programme will be iteratively designed through several in-person/online meetings with a multidisciplinar working group. This group will include at least 3 adult patients with asthma, 1 carer of a patient with asthma, 2 researchers, 2 medical specialists, 1 adult education expert, and 2 multimedia experts.
Content will be produced for each module and learning objectives (Milestone 2, Figure 1). Based on the sources of information identified in the T1.2, the information will be retrieved, selected, and adapted to lay language. Additional resources will be identified and adapted to attractive formats, to provide to the attendee if needed. This content will be produced in different formats (short text, simple scheme, audios and videos). The diversity of contents from the “European Patient Ambassador Programme”, developed by the European Lung Foundation, will be used as an example as this self-learning programme was also produced with the help of patients. A short exercise for knowledge self-assessment will be created for each module. At the end of each module, a satisfaction survey regarding time, layout, content, language, and suggestions for improvements will be created.
T2.2. Implementing the self-learning programme
The content produced (T2.1) will be deployed on a web platform. To accelerate the implementation of the programme, existing e-learning platforms used by scientific societies, health institutions and/or health e-learning companies to provide online courses to health professionals will be sought. During the implementation of the content by the multimedia experts, the remaining team will act as beta testers of the platform to detect most errors/bugs during this stage.
T2.3. Usability of the self-learning programme
The usability of the implemented self-learning programme will be assessed. At least 8 patients will be recruited from the ConectAR network (convenience sample) to allow the detection of 50-80% of the issues 18. Use of questionnaires such as the User Experience Questionnaire and System Usability Scale are anticipated. Reported technical issues will be then solved (T2.2). At the end of this task, we expect to have the online self-learning programme with three modules implemented, available online, free of charge, upon registration (Milestone 3, Figure 1) and ready to be tested with a large audience.
Task 3. Evaluation of the self-learning programme
This task aims to assess the feasibility, acceptability and impact of the self-learning programme. The self-learning programme will be made available to patients/carers with asthma (T3.1), after three months its feasibility and acceptability assessed (T3.2) and after 6 months its real- life impact evaluated. This task has the duration of 9 months.
T3.1 Delivering the self-learning programme
The self-learning programme will be available to the target audience - adult patients with asthma and carers. Minors (patients or carers) will not be eligible as contentes will not be adapted to the paediatric population. To reach this audience, the self-learning programme will be disseminated across all ConectAR network, Portuguese patients’ and carers’ associations (APA, Respira, ANCI - Associação Nacional Cuidadores Informais), and scientific societies and networks (SPAIC, SPP, GRESP, Fundação Portuguesa do Pulmão, Rede de Especialistas em Asma Grave/REAG).
The programme will also be disseminated to the larger community through social media and healthcare units. The self-learning programme will be free of charge upon registration, which will include profile (patient/carer/health professional, other), age and gender. Attendees will be additionally asked to submit their learning needs and their interests in health research before starting and will be asked to participate in the impact evaluation study (not mandatory) (T3.3). The programme will be flexible so attendees can choose the order of the modules, can stop, and start whenever they want and can see their progress continually. A certificate will be issued at the end of each completed module.
T3.2. Assessing feasibility, satisfaction, and acceptability
The self-learning programme will be evaluated three months after being available. Feasibility will be assessed based on the following outcomes: (i) characterisation of patients’ profile (patient/carer, age, gender); (ii) retention rate (the number of participants who concluded at least one module divided by the number of registered participants); (iii) completion rates (the number of attendees who completed the self-learning programme divided by the number of registered participants). The attendees’ satisfaction (time, layout, language, areas of improvement) will be analysed. Acceptability will be assessed through a focus-group interview with at least 6 participants with different profiles and behaviours (patients/carers; completers/non completers). A semi-structured guide will be used to explore participants’ perspectives about the appropriateness of the programme contents, structure and procedures; its impacts, benefits and disadvantages; barriers and facilitators for participation.
T3.3 Evaluating impact
An observational, prospective study will be conducted. Ethical approval will be obtained (please see section Ethics and diffusion activities). All the attendees of the self-learning programme will be asked to fill in a questionnaire on 1) the knowledge, 2) confidence in the ability to use information/explain to others, and 3) intention to be actively involved in research, at three time-points: before starting, immediately after and six months after completing the programme. Questionnaires such as Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire (KASE-AQ) 19 will be used.
Based on patients’ feedback, gathered during T3.2 and T3.3, a report with the improvements needed in the self-learning programme will be produced to inform future updates. The results of the feasibility and impact of the self-learning programme will be disseminated in the format of a scientific publication (abstract or original article) (Milestone 4, Figure 1).
Task 4. Setup, coordination and project dissemination Setup and coordination are critical for the management of the project, from its design to dissemination. To ensure the inclusion of the patients’ perspectives, both patients with asthma and carers are taking an active role as members of the study steering committee. Also, the involvement of patients will enable the creation of contente in clear, user-friendly and non-technical language, to ensure that the self-learning programme is accessible to the lay public. An example is already in place in this proposal, as patients and carers of the research team built the public summary of this proposal. The dissemination of the results of the project includes the writing and production of scientific outputs and diffusion to the general public. This task runs throughout the entire project.
The management of this project will include a Steering Committee, composed of three patients with mild, moderate- to-severe and severe asthma and a carer of a minor with asthma as well as three researchers. Advisory board will include an expert on adult education, an expert on communication science and digital media, an expert on asthma and an expert on mHealth/e-Health solutions.
The Steering Committee will make strategic decisions about the project, such as management of the scientific and financial components of the project; management of services providers agreements; submission to ethical committees, and will organise monthly meetings. Their main role will be to guarantee the smooth running of the project, and efficient coordination between team members to achieve project goals.
A kick-off meeting will be organised, to establish general procedures and to discuss the roles of the team members throughout the project. During this task it is expected to achieve 1) the preparation and submission of applications to the ethics review board; 2) the implementation of a collaborative shared online folder for the project team; 3) the definition of the requirements for the services that will be purchased, namely for the implementation of the self-learning programme; 4) dissemination activities, including at least one original paper and results to be presented in at least two scientific meetings.
ETHICS AND DIFFUSION ACTIVITIES
For the consensus process, in which participants will be a group of patients with asthma and carers involved as co-researchers in health research, concerns with privacy and personal data protection will be considered. Ethical approval will not be needed as patients and carers in this group act as equal partners with academic researchers and healthcare professionals, instead of being subjects under investigation. Personal data, namely name and email contact, will be collected and securely stored/managed in a centralised database at the host institution following the European General Data Protection Regulation (GDPR-EU 2016/679) standards.
The feasibility and impact studies (Task 2 and 3) will be conducted according to the Declaration of Helsinki and Oviedo Convention. Patients’ personal data will be collected and treated according to the Portuguese law on Data Protection (Law number 67/98, October 26th) and the GDPR, executed and enforced in Portugal by Laws 58 and 59/2019. Specifically, to conduct these studies, ethical approvals will be obtained. Written informed consents will be obtained prior to any data collection from patients in accordance with GDPR requirements. Informed consents will include information to the participants regarding the types of personal and sensitive data that will be collected, how it will be processed, for what purposes and what security measures will be in place to provide for their privacy, as well as the conservation period or their elimination, after the project ends.
There are no risks anticipated for patients in relation to the participation in this study. The privacy of the patients/carers in both studies will be ensured by pseudo-anonymisation techniques, which generate an anonymous univocal numeric code for each subject and the coding key will be encrypted and securely stored and managed, separately from patients’ personal and health-related data. All data analyses will be performed within the pseudo-anonymised versions of the original databases. The computers that contain patient personal data will be subjected to password protection only known by the Principal Investigator (PI) and the Co-PI. The storage of patients’ data will be managed through a centralised database structured in order to guarantee privacy and personal data protection. Only the PI will have access to the original database. This database will be saved on a server of the host institution, with daily backups and firewall protection. All paper documents that cannot be scanned in time, and that include personal data, will also have protection measures with physical access control provided to only authorised researchers.
The project’s results, including the self-learning programme, will be disseminated among patients, the general public, researchers, health professionals, decision-makers and educators. The project will use its own social media channels and other media (e.g. press communications) to attractively diffuse the self-learning programme to the public. Scientific outputs of the project include at least one original paper and results to be presented in at least two scientific meetings.
EXPECTED RESULTS AND POSSIBLE LIMITATIONS
The expected results at the end of each task are explained in the Methods and analysis section. By the end of this project, we expect to have implemented the first high-quality, free-of-charge, online self-learning programme on asthma and health research, adapted for adult patients with asthma and their carers. The programme will be designed upon reliable sources of information and based on patients/carers’ needs and preferences. It has the additional advantage of being available for patients/ carers not only from Portugal, but also from other Luso-phone countries. This attractive self-learning programme will empower patients to increase their knowledge, develop new skills and have the opportunity to learn about research. Ultimately, it will introduce the knowledge patients/ carers need to represent themselves and influence health policies in Portugal successfully.
Despite the benefits for patients and society, some difficulties are expected during this project’s implementation.
As stated before, one of the major barriers to the PPI in health research is the frustration and the time constraints, namely the time patients and carers need to invest in training and support. To overcome these barriers, researchers and healthcare professionals involved in this project are made aware of the need to improve specific competencies 20 namely, participation, communication, use of common vocabulary, teamwork and conflict/tension management. To improve these soft skills and increase awareness on how to address patients/carers, the present self-learning programme will also be available for healthcare professionals and researchers.
A selection bias is also expected since this programme is expected to attract younger patients/carers and with higher levels of education and digital literacy. Other training formats, such as group sessions, networking and shared learning with peers will need to be explored later to reach older patients and/or with lower literacy levels.
Another limitation is that this programme focuses specifically on asthma. In future, the methodology of the present project can therefore be replicated for other chronic respiratory diseases, such as chronic obstructive pulmonary diseases and lung cancer.