INTRODUÇÃO
Marula (Sclerocarya birrea (A. Rich.) Hochst.) is a fruit-bearing tree native to the semiarid savanna of South Africa (Kugedera, 2019; Mouk et al., 2007), it is a deciduous, dioecious species of the family Anacardiaceae (Midgley et al., 2010) and very important in several regions of Africa due to its wide range of uses, mainly as a food for humans and animals and as medicine (Gouwakinnou et al., 2011).
However, seed dormancy is an obstacle for marula production and hinders the species propagation. Marula seeds do not germinate easily, as they become dormant after fruit harvest and may remain inactive or dormant for more than six months after falling from the canopy, which cause a slow and uneven seed emergence. The marula seed is coated by a rigid, woody, lignified endocarp that prevents the growth and development of the embryo and limits the entry of water and oxygen into the seed (Carvalho & Nakagawa, 2012).
The use of pre-germination treatments, such as immersion in water, chemical and mechanical scarification, and growth regulators, are strategies for accelerating and standardizing the germination of seeds, as well as for subjecting them to storage periods, which is a practical method often used for seed dormancy breaking (Marcos Filho, 2015). According to this author, storing seeds overcome all causes of dormancy, such as imbalances between growth-promoting and -inhibiting substances, mechanical resistance of the seed coat, embryo dormancy, impermeability to gases and water, and combinations of these factors.
Seed quality is a significantly important factor for obtaining the expected yield, and seed storage is an essential practice for maintaining the physiological quality and preserving the viability and vigor of seeds for the following sowing season (Azevedo et al., 2003).
In this context, the objective of the present work was to evaluate the effect of storage periods on dormancy breaking and physiological quality of marula seeds.
MATERIAL AND METHODS
The experiment was conducted at the Laboratory of Plant Physiology of the Department of Agricultural Sciences (DCA) of the State University of Montes Claros (UNIMONTES) in Janauba, MG, Brazil. Marula seeds were obtained from mature fruits of plants with approximately 10 meters in height, planted in 2009 at the Experimental Farm of the UNIMONTES. Fruits on the ground with yellowish skin were collected, as they had probably reached the physiological maturation stage.
The experiment was conducted in a completely randomized design, with five treatments and four replications; each replication consisted of 25 seeds. The treatments consisted of five storage periods: 10 (control), 184, 244, 306, 367, and 428 days after fruit harvest. The collected fruits were sent to the Laboratory of Plant Physiology for fruit pulping, in which the mucilage adhered to the seeds was removed by friction in a 5-mm mesh sieve with subsequent washing in running water for five minutes. The seeds were then shade dried under room conditions.
The seeds were evaluated for water content, 1000-seed weight, seed biometry, seedling emergence, emergence speed index, and percentages of hard seeds, dead seeds, and abnormal seedlings.
The seeds were evaluated for moisture 10 days after drying and before applying the treatments, using the oven method at 105±3 °C for 24 hours; the results were expressed as percentages, as established in the Rules for Seed Analysis (Brasil, 2009).
Seedling emergence was determined in the laboratory under room conditions (26±2 °C). Seeds were sowed to a depth of 5.0 cm in plastic trays containing washed sterilized sand, which was moistened with the amount of water equivalent to 60% retention capacity (Brasil, 2009). Evaluations were carried out 70 days after sowing by counting normal seedlings, considering seedlings that presented cotyledons above the substrate surface as normal; abnormal seedlings and hard seeds were also counted; the results were expressed as percentages.
Emergence speed index (ESI) was evaluated by daily counting the number of emerged seedlings up to 70 days after sowing, and then calculated using the formula proposed by Maguire (1962).
Seed biometry (length, width, and diameter) was measured with the aid of a digital caliper (0.01 mm), using four 100-seed replications; the results were expressed in cm. The 1000-seed weight was determined using eight 100-seed replications of pure seeds; the seeds of each replication were weighed in a precision balance (0.0001 g).
The results were subjected to analysis of variance by the F test at 5% probability after meeting the basic assumptions (normal distribution of errors and homogeneity of variance). The F test was significant; thus, the means were subjected to regression analysis, and the estimates of the parameters were evaluated by the t test at 5% significance, choosing the most appropriate models based on biological dynamics. The statistical analyses were carried out using the Statistical Analysis System R.
RESULTS AND DISCUSSION
The initial water contents of the marula seeds after storage at 6, 8, 10, 12, and 14 months after fruit harvest was 7.6%. Seed water contents were relatively low after harvest and after the storage periods, which denotes the greater reliability of the results obtained in the seed physiological quality tests. This is an important factor, since seed longevity is directly connected to water content, which directly affects physiological processes and may decrease seed quality, thus affecting the vigor and even the germination potential of the seeds (Marcos Filho, 2015).
The mean 1000-seed weight found was 3,895.7 g, regarding the biometric characteristics width, length, and diameter, the Person's correlation showed no significant results, with means of 24.3±1.98, 25.2±2.21, and 16.1±2.01 cm, respectively.
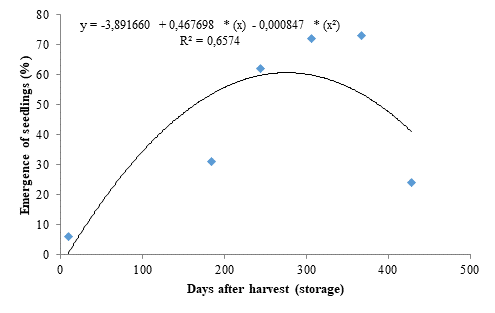
The storage periods had significant effect on all evaluated variables. The seedling emergence data fitted to a quadratic polynomial regression model (Figure 1), with seeds stored for 307 days resulting in a seedling emergence percentage of 60.67% (Figure 1), representing a 911% increase when compared to the control, denoting that a storage period of 277 days is efficient for dormancy breaking in marula seeds; however, the seedling emergence decreased from this period onwards, reaching 24% in seeds stored for the longest period (428 days).
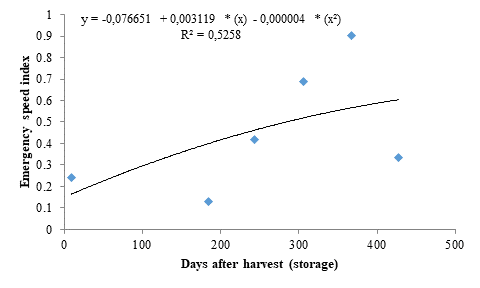
The data of emergence speed index (ESI) fitted to a quadratic polynomial regression model (Figure 2). Seeds stored for 390 days resulted in higher ESI (0.68), representing an increase of 1600 % when compared to the control, denoting the dormancy breaking of the seeds. However, the ESI decreased from this period onwards, reaching 0.59 in seeds stored for 428 days.
The decreases found for seedling emergence and ESI after reaching the peak may be due to an imbalance between growth-promoting and -inhibiting substances and the resuming of embryo dormancy due to the mechanical resistance of the seed coat, which causes impermeability to gases and water after the ideal storage period. According to Moyo et al. (2009), a 12-month storage period under ideal climate conditions favored the germination of marula seeds by dormancy breaking, which are consistent with the results found in the present study.
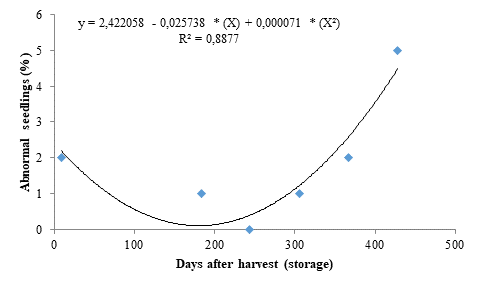
The percentage of abnormal seedlings presented similar results to those found for the other evaluated variables, also fitting to a quadratic polynomial regression model (Figure 3). It decreased as the storage period increased up to 182 days, reaching 0.087 %, but increased from this period onwards, reaching 5 % in seeds stored for 428 days. This result may be due to deterioration of the seed embryo caused by the storage time.
Considering that occurrences of abnormal seedlings indicate impaired seed physiological quality, the storage of seeds for more than 428 days may have accelerated metabolic reactions in the seeds, causing inadequate emergence. According to Carvalho and Nakagawa (2012), seeds with high levels of deterioration have less energy available for the restoring process of injured tissues, which becomes inefficient, significantly affecting the germination process by preventing or delaying it or causing the growth of abnormal seedlings.
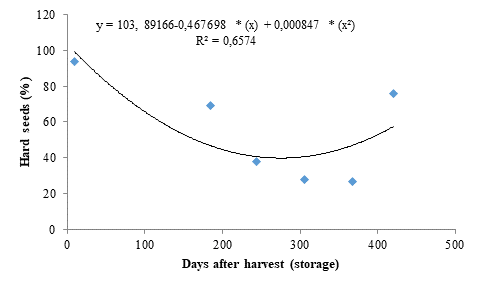
The hard seed data (Figure 4) fitted to a quadratic polynomial regression model and presented dynamics inversely proportional to seedling emergence (Figure 1). A percentage of 94 % of hard seeds was found for the initial storage period. However, the storage period of 277 days was efficient in significantly reducing the percentage of hard seeds, explaining the high seedling emergence percentages found for these seeds (Figure 1). However, the percentage of hard seeds increased from this period onwards, reaching 76 %.
The decreases found for percentage of hard seeds may be connected to integument wear caused by drying, which makes the seeds more permeable to water and gases, favoring embryo maturation due to a balance between growth-promoting and -inhibiting enzymes. The increase in percentage of non-germinated seeds after 368 days of seed storage may be due to a decrease in seed vigor, caused by the deterioration progress, which can directly affect the seed performance by decreasing energy production and impairing biosynthesis, thus affecting their potential for storage.
Decreases in seed physiological potential over time are not limited to decreases in germination potential, but also to a slower germination process and increased sensitivity to environmental stress, which characterize decreases in seed vigor (Marcos Filho, 2015). Thus, the beginning of the degenerative process triggers the degradation of membrane systems, reducing the seed resistance to rapid water influx and leading to imbibition and severe damage to cell metabolism (Marcos Filho, 2015).
CONCLUSIONS
Germination rates are improved by advancing the storage period for up to 367 days. Storing marula seeds under room conditions (26±2 °C) for 367 days after harvest is recommended for a better physiological quality of the seeds.
Overall, the results of this study provide a considerable advance in our knowledge of the physiological quality and dormancy overcoming in Sclerocarya birrea seeds.