INTRODUCTION
Horticultural production for industry is mainly based on monoculture systems with a high degree of intensification and technical intervention. At the soil level, these practices result in biodiversity imbalances, loss of fertility, increased erosion and surface runoff. The low plant diversification reflects in the structure of soil microbial communities, disfavouring plant-beneficial populations and facilitating the emergence and progression of soil-borne phytopathogens, which compels producers to excessive use of phytopharmaceuticals. All this tends to aggravate in the climate change scenario that is already a reality. Therefore, there is an urgent need to adopt alternative and more eco-friendly production strategies.
Cover crops have been considered as a promising option for the sustainable production of agricultural systems. In intensive monoculture systems, the introduction of cover crops during the fall-winter period preceding the main crop of the agricultural year may positively influence the beneficial soil microbiota, enhancing soil health and contributing to mitigate the consequences of soil degradation. This has been addressed in projects MaisSolo and HortiCover, particularly referring to intensive horticultural systems in the Portuguese region of Ribatejo. The objective of the present work was to evaluate the response of soil microbiological indicators to the introduction of cover crops in these systems.
MATERIAL AND METHODS
The evaluations were carried out in the scope of project MaisSolo, in two farms located in Ribatejo (São João de Brito and Quinta do Manique) between 2018 and 2020 (Table 1). In each farm, four different modalities (0.5 ha plots) were installed during the fall-winter period: 1) biodiverse mixture of legumes and grasses, including clovers inoculated with rhizobia; 2) Lolium multiflorum (annual ryegrass); 3) Raphanus sativus (forage turnip) for biofumigation; and 4) no cover crop (control).
Table 1 Location, soil type and main crops in the field trials
| Farm | Location | Soil type | Main crop (2018/2019/2020) |
|---|---|---|---|
| São João de Brito | Golegã | Fluvisol | Potato/Maize/Maize |
| Quinta do Manique | Vila Franca de Xira | Fluvisol | Tomato |
Samples of rhizospheric soil from end-of-cycle plants were collected and the following indicators were evaluated: enzyme activities (dehydrogenase, alkaline phosphatase, β-glucosidase), abundance of total culturable bacteria, abundance of free-living nitrogen-fixing aerobic bacteria, abundance of phosphate-solubilizing bacteria, activity of phytostimulating microorganisms, and abundance of symbiotic nitrogen-fixing bacteria (rhizobia). The methods are in Table 2.
Table 2 Methods used in the evaluation of indicators
| Indicator | Method* |
|---|---|
| Dehydrogenase activity | Spectrophotometric quantification of triphenyl formazan formed from the reduction of 2,3,5-triphenyltetrazolium chloride (Menino et al., 2021, adapted from Casida et al., 1964) |
| Alcaline phosphatase activity | Spectrophotometric quantification of p-nitrophenol formed from p-nitrophenyl-phosphate (adapted from Tabatabai, 1994) |
| β-Glucosidase activity | Spectrophotometric quantification of p-nitrophenol formed from p-nitrophenyl-β-glucoside (adapted from Tabatabai, 1994) |
| Abundance of total bacteria (heterotrophic) | Dilution spread-plate method (CFU counts in generic agar medium) (Zuberer, 1994) |
| Abundance of free-living aerobic nitrogen-fixing bacteria | Dilution spread-plate method (CFU counts in N-free agar medium) (Martinez-Toledo et al., 1985) |
| Abundance of phosphate solubilizing bacteria | Dilution spread-plate method (CFU forming solubilisation haloes on agar medium supplemented with Ca3(PO4)2) (Castagno et al., 2011) |
| Activity of phytostimulating microorganisms | Stimulation of Arabidopsis thaliana growth (fresh weight) upon inoculation with extracted soil microbiota |
| Abundance of symbiotic nitrogen-fixing bacteria (rhizobia) | Most Probable Number by the plant-infection method (Vincent, 1970) using Trifolium suaveolens as host plant |
* CFU, colony forming units.
RESULTS AND DISCUSSION
Several soil microbiological indicators were assessed in two field trials in the scope of project MaisSolo, aiming to evaluate the effects of cover crops on soil microbiology. In order to obtain the general response tendency of each indicator, the sets of data from both fields over time (whenever available) were here considered for the analysis.
The results showed a general increase in soil enzyme activities when using the biodiverse mixture of legumes and grasses or annual ryegrass as cover crops, relative to soils without cover (Figure 1.A). The exception was β-glucosidase activity with annual ryegrass. Soil enzymes play an essential role in the degradation, mineralization and immobilization of nutrients in soil, and can be used as dynamic bioindicators of soil quality and health. In this study, we evaluated three enzyme activities that participate in organic matter degradation and nutrient cycling in soils. Dehydrogenases are a wide and diverse group of intracellular enzymes involved in microbial respiration pathways, and the measurement of their total activity reflects the metabolic activity of the soil microbiota (Makoi & Ndakidem, 2008) and indicates about the potential to oxidise soil organic matter (Alkorta, et al., 2003). Phosphatases are a broad group of extracellular enzymes that play a key role in the mineralization of organic phosphorus and are considered as good indicators of soil fertility (Makoi & Ndakidem, 2008). β-glucosidases act on the hydrolysis of β-glucosyl residues (including the final step in cellulose hydrolysis) to release β-D-glucose, which can be an energy source for soil microorganisms (Alkorta et al., 2003). Thus, our results on the evaluation of soil enzymes indicate that the introduction of cover crops in these systems, particularlly the biodiverse mixture of legumes and grasses and annual ryegrass, result in higher soil microbiological activity and increased potential for nutrient cycling.
Cover crops also positively influenced beneficial rhizospheric microorganisms, including several groups of plant growth promoters (Figure 1.B-D). The abundance of phosphate solubilizing bacteria increased with the biodiverse mixture of legumes and grasses and with annual ryegrass, compared with controls without cover crops (Figure 1.B). These bacteria have the ability to remove phosphorus adsorbed to soil particles and make it available to plants. Our results indicate a greater capacity to solubilize soil-immobilized phosphorus, which is particularly relevant in soils with low levels of bioavailable phosphorus and may also improve the utilization efficiency of phosphorus fertilizers.
The introduction of cover crops, especially annual ryegrass, increased the phytostimulating activity of rhizospheric microorganisms (Figure 1.C). Several microorganisms are capable of producing molecules (phytohormones) that stimulate root growth and plant development. Phytohormones of microbial origin affect the metabolism of endogenous growth regulators in plants and may improve their performance under different types of stress (Egamberdieva et al., 2017). By stimulating plant growth and stress resistance, this will contribute to more resilient ecosystems.
The symbiotic nitrogen fixing bacteria, generically designated as rhizobia, are able to fix atmospheric nitrogen in symbiosis with legume plants, playing a key role in the nitrogen nutrition of the host and the sustainable enrichment of the soil in nitrogen. The evaluation of the rhizobial abundance in these soils revealed very low native populations. However, important increases were observed following the introduction of the mixture of legumes and grasses, in which the clovers had been inoculated with rhizobia (Figure 1.D). This result shows the importance of introducing inoculated legumes to enrich the soil with these bacteria.
Free-living (non-symbiotic) nitrogen-fixing bacteria can also contribute to the enrichment of the rhizosphere in nitrogen. These bacteria were naturally abundant in these soils (107-108 colony forming units/g soil) and, as with total bacteria, were not affected by the introduction of cover crops (Figure 1.B).
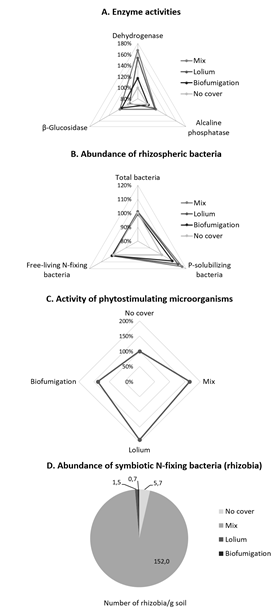
Figure 1 Soil microbiological indicators evaluated in the field trials. Except for D, results are presented as percentage of the control (no cover). A, B: mean values of the determinations in both fields in 2018-2020, at the end of cycle of the main crop. C: São João de Brito in 2018, at the end of cycle of the main crop. D: mean values of the determinations in São João de Brito in 2019-2020, at the end of cycle of the cover crops and main crop. No cover - control without cover crops; Mix - biodiverse mixture of legumes and grasses; Lolium - annual ryegrass; Biofumigation - forage turnip.
CONCLUSIONS
In two horticultural production farms, the introduction of cover crops, especially the biodiverse mixture of legumes and grasses and annual ryegrass, increased soil microbiological activity and favoured several groups of plant-growth promoting microorganisms, including phosphate solubilizing bacteria, phytostimulating microorganisms and symbiotic nitrogen-fixing bacteria. These indicators are positively correlated with the equilibrium and robustness of the soil microbiome, pointing to a favourable evolution of the soil status. The evaluations are now proceeding in project HortiCover, which develops biodiverse cover mixtures based on selected ecotypes, optimally adapted to the cultural systems and to climate change.














