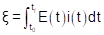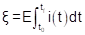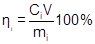INTRODUCTION
Rare earth elements (REEs) are composed of 17 chemical species, which are comprised of the lanthanide series and the elements yttrium and scandium (Couto et al., 2020). These species present many important applications in green energy production and high technology areas (Balaram, 2019). In this sense, REEs are essential species for the development of a decarbonized society.
REEs reserves are available in different countries, such as China, Brazil, and Australia. However, China holds more than 90% of REEs world production, and this monopoly condition enables China to control the REEs exportation. This circumstance represents a sign of alert to the global consumers, as REEs are critical raw materials and their use is increasing (Cánovas et al., 2019; EC, 2020).
REEs extraction is mainly conducted via hydrometallurgical process or by ion adsorption clays. These techniques can achieve high levels of purity in REEs production. However, these extraction processes can result in higher energy consumption and several environmental damages (Jha et al., 2016).
Electromining is an eco-friendly electrokinetic process to remove metallic ionic species from soils (Pires et al., 2022). To that end, electrodes are arranged in a migrational cell, and due to an electric field action, cations migrate to the cathode, and anions towards the anode (Acar et al., 1995).
Considering the disadvantages of REEs production via conventional process, and also taking into account the risk of disruption of supply of these species, the use of eco-friendly techniques to remove REEs is crucial. In this sense, the aim of the present work was to evaluate the removal of lanthanum and neodymium from soils applying the electromining technique using green electrolytes in low concentrations.
MATERIAL AND METHODS
The soil used in this work was collected from the north of Brazil. Table 1 gathers some information about soil composition. X-ray fluorescence was performed to quantify species in higher concentrations. To quantify lanthanum (La3+) and neodymium (Nd3+) ions, a microwave assisted acid digestion was conducted, followed by Inductively coupled plasma (ICP-OES).
Table 1 Soil composition
| Soil composition (%) | ||||
|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | Nb2O5 |
| 60.7 | 25.6 | 2.0 | 1.9 | 1.3 |
| SnO2 | ZrO2 | Ta2O5 | K2O | MnO |
| 1.3 | 0.3 | 0.1 | 0.1 | 0.1 |
| REE (mg kg-1) | ||||
| La | Nd | |||
| 2.5 | 2.8 | |||
To conduct the electromining experiments, around 300 g of soil was loaded into the migrational cell bed (Figure 1). The titanium electrodes were located at the end of the migrational cell and were connected to the power supply (Agilent - E3645A). To monitor the anolyte and catholyte pH, pH meters (Hanna Instruments - HI1083) were inserted in the electrolyte chambers, as shown in Figure 1. Aliquots of electrolyte solutions were collected to quantify (ICP-EOS) the extraction of La3+ and Nd3+ ions. The electric current was monitored each 24 h.
The experiments were conducted applying an electric field of 1.0 V cm-1 and using citric and oxalic acid as electrolytes at 0.10 mol L-1 (Pires et al., 2022). All solutions were prepared with ultrapure water (Direct Q - Merck Millipore).
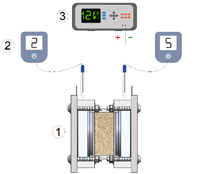
Figure 1 Experimental apparatus used in the electromining experiments: 1) migrational cell, 2) pH meter, 3) power supply.
The energy consumption (ξ), in W h, of the electromining process was given by
where E (V) represents the electric potential applied and i (A) is the electric current of the electromining process. Considering that the experiments were conducted using the potentiostatic method, E(t) is constant during the experiments. The average electric current was obtained by fitting discrete current values, which were monitored every 24 h. In this sense, Eq.(1) can be rewritten as
The electromining efficiency to the i-th species (ηi) was obtained according to
where Ci (mg L-1) is the concentration of the i-th species obtained at the end of experiments. V (L) is the volume of the electrolyte chambers, and mi (mg) is the initial mass of i-th species in the soil.
RESULTS AND DISCUSSION
The electromining experiments were conducted for 240 h. Migrational flow towards the anodic chamber was not observed. Hence, all results were shown regarding catholyte. The experiment denoted by EM-01 was conducted using citric acid as electrolyte, and EM-02 was conducted with oxalic acid.
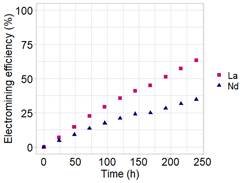
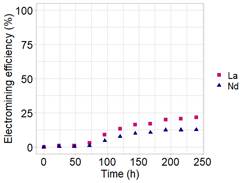
The profile of the electromining efficiency in the cathodic chamber of La3+ and Nd3+ for electromining experiments EM-01 and EM-02 are shown in Figure 2 and Figure 3, respectively.
In each experiment, similar behavior of REE ions removal was observed. However, when comparing these experiments, the electrolyte effect on the electromining efficiency can be seen, as EM-01 presented removal values around 63% and EM-02 achieved values of 21% of extraction (Figure 2 and Figure 3), considering the 10 days of the experiments.
Table 2 Values of electromining efficiency, energy consumption, and pH of catholyte
| pKa1 | 3.15* | Citric acid | EM-01 | 1.25* | Oxalic acid | EM-02 | |||
| pH | 1.91 ± 0.05 | Anolyte | EM-01 | 1.09 ± 0.06 | Anolyte | EM-02 | |||
| pH | 2.31 ± 0.16 | Catholyte | EM-01 | 2.60 ± 0.89 | Catholyte | EM-02 | |||
| ξ (W h) | 12.56 | EM-01 | 33.83 | EM-02 | |||||
| η (%) | La3+ | Nd3+ | 63.20 | 34.73 | EM-01 | 21.56 | 12.72 | EM-02 |
As oxalic acid presents a higher dissociation capacity (pKa), this condition, associated with the low values of pH in the anodic chamber (Table 2), may have favored the desorption and leaching of other species from the soil. In this sense, a higher number of species immersed in the electric field can disfavor the individual migration of species (Pires et al., 2022). For this reason, citric acid presented the best electromining efficiency. Moreover, according to the removal tendency of La3+ and Nd3+ (Figure 2), the extraction could achieve higher values if the experiment time was extended.
Concerning the energy consumption, EM-01 required 12.6 W h to remove the species (Table 2). On the other hand, EM-02 consumed almost three times more energy and presented lower removal. This behavior can be associated with the strength of the acid used (oxalic acid), which can release more species to the solution, increasing the electric current and, hence, the energy consumption.
CONCLUSIONS
This work presented an evaluation of the use of two different green electrolytes in electromining experiments. Citric acid presented the higher removal efficiencies, achieving 63.2% of La3+ and 34.7% of Nd3+ in 10 days of the experiment. Furthermore, according to its extraction tendency, the removal of REEs ions could be performed for more time, as the experiment was finished before the stabilization of REEs removal.