Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.42 no.4 Lisboa dez. 2019
https://doi.org/10.19084/rca.17865
ARTIGO
Agronomic performance of lowland rice plants promoted by beneficial microorganisms
Desempenho agronômico de plantas de arroz irrigado promovido por microrganismos benéficos
Israel M. Sousa1,*, Adriano S. Nascente2, Marta Cristina C. Filippi2 and Anna Cristina Lanna2
1Universidade Federal de Goiás, Escola de Agronomia, Programa de Pós Graduação em Agronomia, Avenida Esperança, s/n, Chácaras Samambaia, CEP 74690-900 Goiânia GO, Brasil
2Embrapa Arroz e Feijão, Rodovia GO-462, Km 12, CEP 75375-000 Santo Antônio de Goiás GO, Brasil
(*E-mail: israelmmendes128@gmail.com)
ABSTRACT
This work aimed to determine the effect of application forms of growth promoting microorganisms on tropical lowland rice plants development, in two experiments. EI was performed in completely randomized design (CRD) in factorial scheme 7x3+1. Treatments were six rhizobacteria: BRM32109 and BRM32110 (Bacillussp.); BRM32111 (Pseudomonas fluorescens); BRM32112 (Pseudomonassp.); BRM32113 (Burkholderia pyrrocinia); BRM32114 (Serratiasp.) and Trichoderma asperellumpool fungus (UFRA.T06+UFRA.T09+UFRA.T12+UFRA.T52) with three application forms (microbiolized seed; seed + soil drenched with microorganism at eight and 15 days after sowing (DAS) and seed + plant sprayed with microorganism at eight and 15 DAS), and control. In EII, microbiolized rice seeds were sowed on test tubes in CRD. Treatments were the same six rhizobacteria of EI and control (water). Isolates BRM32110, BRM32111, BRM32112 and BRM32113 improved gas exchange in lowland rice plants. For biomass production, there were interactions between types of microorganisms and application forms. In general, microbiolization + plant sprayed was the most efficient (10.3%) to increase dry matter biomass of rice shoots. Stoodout BRM32109, BRM32111 and BRM32113, which, increased, on average, 19% of dry rice biomass when compared to the control plants. Root length of rice seedlings treated with microorganisms was, on average, 89% higher than control plants.
Keywords: Oryza sativaL., Growth promoting, Plant growth promoting rhizobacteria, Sustainable production.
RESUMO
Objetivou-se determinar o efeito de formas de aplicação de microrganismos promotores de crescimento no desenvolvimento de plantas de arroz irrigado tropical, em dois experimentos. EI foi realizado em delineamento experimental inteiramente casualizado (DIC) em esquema fatorial 7x3+1. Tratamentos consistiram de seis isolados de rizobactérias: BRM32109 e BRM32110 (Bacillussp.); BRM32111 (Pseudomonas fluorescens); BRM32112 (Pseudomonassp.); BRM32113 (Burkholderia pyrrocinia); BRM32114 (Serratiasp.) e pooldo fungo Trichoderma asperellum(UFRA.T06+UFRA.T09+UFRA.T12+UFRA.T52) com três formas de aplicação aplicação (semente microbiolizada; semente microbiolizada + solo regado com microrganismo aos oito e 15 dias após a semeadura (DAS) e semente microbiolizada + pulverização do microrganismo na planta aos oito e 15 DAS), e um tratamento controle. Em EII, sementes de arroz microbiolizadas foram semeadas em tubos de ensaio em DIC. Tratamentos foram os mesmos seis isolados de rizobactérias utilizados no EI e um controle (água). Os isolados BRM32110, BRM32111, BRM32112 e BRM32113 melhoraram as trocas gasosas nas plantas de arroz irrigado. Para produção de biomassa, houve interações entre tipos e formas de aplicação dos microrganismos. Em geral, microbiolização + pulverização do microrganismo foi mais eficiente (10,3%) em aumentar a biomassa seca da parte aérea das plantas de arroz. Destaque para BRM32109, BRM32111 e BRM32113, os quais aumentaram, em média, 19% a biomassa seca das plantas quando comparado com o tratamento controle. Comprimento radicular de plântulas de arroz tratadas com os microrganismos foi, em média, 89% maior que o das plantas controle.
Palavras-chave: Oryza sativaL., Promoção de crescimento, Rizobactérias promotoras de crescimento vegetal, Produção sustentável.
INTRODUCTION
Rice (Oryza sativaL.) is the staple food for almost four billion people worldwide (Kumar and Ladha, 2011). According to the United Nations Organization (2017), it is estimated that the growing population is 1.10% per year (approximately 83 million people born per year). Therefore, rice production should increase, in a sustainable way, to attend the global demand for food (Nascente et al., 2017a). The sustainability vision encompasses aspects such as, reduction of the use of synthetic inputs and increase of the use of microbial products, that are ecologically friendly, minimize production costs and risk of negative environmental impact, what hence ensures quantity and quality on food production (Mattos et al., 2006).
It is known that beneficial microorganisms promote effects on plant growth and development in agriculture. They are a sustainable complement to increase efficiency in food production (Isawa et al., 2010). Plant growth promoting rhizobacteria (PGPR) are the most used microorganisms in agriculture and, these microorganisms act directly and indirectly on plant growth (Ahemad and Kilbret, 2014). Such as PGPR, fungi species are belonging to the Trichodermagenus has been studied and also provide improvements in growth and crop yield (Sousa et al., 2018). Besides that, these beneficial microorganisms improve uptake of nutrients (Cuevas et al., 2005) and have antagonistic capacity against phytopathogens (Sousa et al., 2018).
Studies performed at Embrapa Rice and Beans selected six isolates of rhizobacteria (BRM32109, BRM32110, BRM32111, BRM32112, BRM32113 and BRM32114) to be used as plant growth promoters (Filippi et al., 2011). After this, researches in controlled conditions showed that these microorganisms promoted significant increases on biomass production and nutrients uptake in lowland (BRS Catiana cultivar) and upland rice (BRS Primavera CL cultivar) genotypes (Nascente et al., 2017a,b), and also improved diseases resistance of upland rice genotypes (Filippi et al., 2011; Sperandio et al., 2017). Besides that, studies carried out at Federal Rural University of Amazônia, selected and tested as growth promotion and biocontrol agents, on greenhouse and field conditions, four isolates of Trichoderma asperellum (UFRA.T06, UFRA.T09, UFRA.T12, UFRA.T52) (França et al., 2015).
Microorganisms act differently in crops and, even, in different cultivars on the same species (Mendes et al., 2018). Therefore, it is important to seek additional pieces of information and advance in knowledge about the use of the microorganisms on tropical lowland rice crop in other cultivars. Thus, this study aimed to evaluate the agronomical performance of tropical lowland rice plants, cultivar BRS A702 CL, treated with beneficial microorganisms, and characterize the effect of the use of these microorganisms on the root growth of tropical lowland rice seedlings, in the same cultivar mentioned above.
MATERIAL AND METHODS
Experiment I - Effect of beneficial microorganisms on the performance of tropical lowland rice plants.
Environment characterization
The experiment was carried out in a greenhouse conditions, on Santo Antônio de Goiás GO, Brazil, between September and December 2017. The soil used was from arable layer (0 - 0.20 m) of a kaolinic, thermic Typic Haplorthox (Santos et al., 2018) with 377, 260 and 363 g kg -1of sand, silt and clay, respectively. Chemical characteristics of the soil were determined according to the methods described by Donagema et al. (2011). Results were: pH (H2O) = 6.1; Ca2+= 78.4 mmolc dm³ -1; Mg2+= 20.9 mmolc dm³ -1; H+ + Al3+ = 12 mmolc dm³ -1; P = 35.9 mg dm³ -1; K+ = 203 mg dm³ -1; Cu2+= 2.4 mg dm³ -1; Zn2+ = 2.9 mg dm³ -1; Fe3+= 39 mg dm³ -1; Mn2+ = 28 mg dm³ -1 and soil organic matter = 24.7 g kg -1.
Three weeks before sowing of lowland rice, BRS A702 CL cultivar, pots with 7 kg capacity were wholly filled with the soil and fertilized with 70 mg dm-3of N (urea), 400 mg dm-3of P2O5 (simple superphosphate) and 200 mg dm-3of K2O (potassium chloride). The moisture of soil, during all the experiment, was monitored daily and soil was kept saturated until the end of the vegetative stage (flag leaf formation on the main stem), and then was maintained four cm of water blade from the ground until the harvest of the experiment.
Experiment design and treatments
The experimental design was completely randomized in factorial scheme 7x3+1, with four replications. Treatments consisted of seven microorganisms: Bacillus sp. (BRM32109 and BRM32110); Pseudomonas fluorescens (BRM32111); Pseudomonas sp. (BRM32112); Burkholderia pyrrocinia (BRM32113); Serratia sp. (BRM32114) and T. asperellum pool (UFRA.T06 + UFRA.T09 + UFRA.T12 + UFRA.T52), with three application forms: microbiolized seed (seed); seed + soil drenched with microorganism at eight and 15 days after sowing (DAS) (seed-soil) and seed + plant sprayed with microorganism at eight and 15 DAS (seed-plant). Additionally, it was included a control treatment with no microorganisms.
Bacterial isolates (BRM32109; BRM32110; BRM32111; BRM32112; BRM32113; BRM32114) used are part of microorganism collection of Embrapa Rice and Beans, and the fungi isolates of T. asperellum pool (UFRA.T06, UFRA.T09, UFRA.T12, and UFRA.T52) are part of fungi collection of Federal Rural University of Amazonia. Biochemical characteristics and taxonomic classification of rhizobacteria BRM32109, BRM32110, BRM32111, BRM32112, BRM32113 and BRM32114 are available in Nascente et al. (2017a) and T. asperellum in Silva et al. (2011).
Seeds microbiolization
Suspension of each bacterial microorganisms (BRM32109; BRM32110; BRM32111; BRM32112; BRM32113; BRM32114) was prepared in nutrient broth from cultures grown in solid medium 523 (Kado and Heskett, 1970), for 24 hours in 28 ºC under constant shaking. The concentration of each suspension was set in a spectrophotometer at an absorbance of 0.5, in a wavelength 540 nm, corresponding to 1x108 colony forming units per mL (CFU mL-1). Rice seeds were immersed in each of these suspensions and, for control, were immersed in water, for 24 hours at 25 ºC temperature under constant shaking, following the methodology proposed by Filippi et al. (2011). For the rice seeds microbiolization with the T. asperellumpool, 0.5 g of each isolate (UFRA.T06, UFRA.T09, UFRA.T12, and UFRA.T52), which was multiplied and preserved in crushed rice leaves, was weighed and mixed with 6.7 mL of white glue solution (1%) and 200 g of rice seeds and shaked according to the methodology proposed by França et al. (2015).
For microorganism application at eight and 15 DAS, suspensions were prepared as described above, on the same concentration (108CFU mL-1). It were applied 100 mL per pot of bacterial and T. asperellumpool suspensions to drenched for treatments seed-soil. Treatments seed-plant were performed in the form of a direct jet, with manual backpack sprayer with a constant pressure of CO2, utilizing a conical nozzle type (TX-VS2), with a volume approximately 100 L ha-1. Control treatment received water.
Management of rice plants
Fifteen rice seeds were sowing per pot of the genotype BRS A702 CL (resistant to the herbicides of Imidazolinones group). Plant emerged six DAS and thinned was done 20 days after emergence (DAE) to keep three plants per pot. During the tillering stage, at 28 DAE, it was performed topdressing fertilization (two grams of ammonium sulfate and one gram of potassium chloride). The second topdressing fertilization (two grams of ammonium sulfate) was carried out at 48 DAE. Weed control was performed manually, together with plants thinning (20 DAE) and there was no need for intervention to control pests and disease.
Gas exchange
It was carried out the following evaluations on the rice plants for gas exchange: photosynthetic rate (A, μmol CO2 m-2s-1); transpiration rate (E, mmol H2O m-2s-1); stomata conductance (gs, mol H2O m-2s-1); internal CO2concentration (Ci, μmol mol-1) and leaf temperature (Tleaf, ºC), determined by a portable gas meter in the infrared region IRGA (LCpro+, ADC BioScientific). Instantaneous carboxylation efficiency (ICE) was calculated as the ratio of A to Ci [(μmol m-2s-1) (μmol mol-1)-1] (Silva et al., 2013). The measurements were taken between 08:30 and 10:30 am at 67 DAE (V6 stage) and 95 DAE (R3 stage).
Samples were taken in the middle third of the first fully expanded leaf (top to base) during the two evaluation periods. Equipment was set up to use concentrations of 370 - 400 mol mol-1CO2 in the air, which is the reference condition used in the IRGA photosynthesis chamber. Photon flux density photosynthetic active (PPFD) used was 1200 μmol [quanta] m-2s-1. Minimum equilibration time set for performing the reading was two minutes.
Biomass production
A sampling of dry matter biomass of rice shoots was performed at 98 DAE (R3 stage), period that 50% of the lowland rice plants were in full flower stage. Therefore, for each treatment, plants were dried in oven 65 ºC until constant weight and, weighed to determine shoots dry matter.
Nutrients content
After dried and weighed, samples of dry matter shoots were send to the laboratory to determine nutrient contents (N, P, K, Ca, Mg, S, Cu, Mg and Zn), according to the recommendations of Malavolta et al. (1997).
Experiment II: Effect of beneficial bacteria on root system development of tropical lowland rice seedlings
Experimental conditions
The experiment was performed in controlled conditions, in April 2018. Rice seeds, BRS A702 CL cultivar, were sowed in test tubes of 15 mL containing water-agar (0.8% w/v), following the methodology proposed by Sperandio et al. (2017). Each test tubes consisted of the one microbiolized seed, with the six rhizobacteria isolates, separately: Bacillus sp. (BRM32109 and BRM32110); P. fluorescens (BRM32111); Pseudomonas sp. (BRM32112); B. pyrrocinia (BRM32113) and Serratia sp. (BRM32114), as described on the experiment I. Experimental design was completely randomized, in which, each test tube represented an experimental unit, in a total of 10 replicates for each bacteria. After that, tubes were taken in a germination chamber with 28 ºC and 12 hours photoperiod. Root seedling length was determined with a scale ruler at 10 DAS.
Statistical analysis
Data obtained from the experiments I and II were submitted to variance analysis and, when significance was detected, means were compared by the LSD test (p<0.05). Additionally, treatments were compared to the control by the Dunnett test (p<0.05). It was used the SAS statistical package (SAS, 1999).
RESULTS AND DISCUSSION
Experiment I - Effect of beneficial microorganisms on the performance of tropical lowland rice plants
Gas exchange
There was no single effect of microorganisms and form of application and nor interaction between these factors for gas exchange at the vegetative stage of rice plants (Table 1). On the other hand, there was a single effect of microorganisms on gs, and there was a single effect of application forms for all variables evaluated, except for foliar temperature (Table 2). There were no interactions. Higher values of gs were observed on the plants treated with BRM32111, BRM32112, BRM32114 and BRM32110. BRM32110 was different from the treatments T. asperllumpool, BRM32109 and BRM32113. For the application forms, seed microbiolization provided the highest values of A and ICE. Seed microbiolization and seed + soil drenched allowed the highest values of gs in tropical lowland rice plants.
BRM32112, BRM32110, BRM32113 and BRM32111 provided increases, on average, in 19.2% on the photosynthetic rate (A) compared to the control treatments in the full flowering stage (R3) (Table 2). Besides that, the ICE of plants treated with BRM32113 isolate (B. pyrrocinia) was significantly higher to the control treatment. The ICE is estimative of the rubisco enzyme activity. Thus, lowland rice plants that presented higher ICE show better ability to overcome the limitation in the CO2diffusion by the stomata and mesophyll and, hence, higher ability to fixing effectively CO2 (Gálmes et al., 2011). Besides, as ICE, photosynthetic rate is also an essential tool in the determination of adaptation and plant response to specific technologies. Increases in plant growth and as a result, increases in grain yield, could be related to increases in the enzymatic activity associated with photosynthesis, potentiated by biotic factors such as the treatment of plants with beneficial microorganisms.
Biomass production
In this variable, stood out isolates BRM32113 (B. pyrrocinia) and BRM32109 (Bacillus sp.) that provided, on average, increase in dry shoot matter of rice plants around 20.5% comparatively to the control plants (Table 3). It is suggested that this increase in dry shoot matter in plants treated with beneficial microorganisms, could be due microorganisms can improve plant root system (Qin et al., 2005), in which promote higher uptake of water and nutrients, allowing better development of plant shoot. Additionally, that increase also can be associated with higher photosynthetic rate and also higher instantaneous carboxylation efficiency showed by plants treated with BRM32113.
It was observed significant interaction in shoot dry matter production of tropical lowland rice plants, between types and application forms of microorganisms tested (Table 3). Seed microbiolization was the most effective application form in the treatment of the tropical lowland rice plants treated with T. asperellumpool and BRM32111. For the BRM32109 and BRM32111, the better application form was seed-soil and, to the BRM32113 was seed-plant. Beneficial microorganisms applied by seed-plant, provided, on average, increase of 10.3% on dry matter shoots compared to the other application forms. It is worth to mention that isolates that promoted higher biomass production showed different behavior regarding application form. To the BRM32113 recommended application by seed-plant, and, to the BRM32109 and BRM32111 application by seed-soil. Nascente et al. (2017a,b) showed similar results about the interaction between forms and types of microorganisms application with lowland and upland rice, respectively.
Nutrients content
Amount of nutrients in shoots of lowland rice plants, treated with beneficial microorganisms, did not differ significantly to the control plants (Table 4). In the other hand, regarding types of beneficial microorganisms, it was observed the difference in the content of K and Zn. Lowland rice plants treated with BRM32111, BRM32112, BRM32113 and BRM32114 stood out for providing increase on the K content in rice shoots of tropical lowland plants. For the Zn, plants treated with T. asperellumpool and BRM32109 showed higher values.
According to Gonçalves (2016), flood irrigation increases soil pH to seven. The low concentration of Zn and the high pH of the soils are the main factors that limit the availability of this nutrient to the plants (Abreu et al., 2007). In this context, Shakeel et al. (2015) identified isolates of bacterial genus Bacillussp., such as BRM32109 (Bacillussp.) used in this study, with the ability to solubilize and improve Zn availability for rice plants. Besides, Silva et al.(2012) showed that three of four isolates of T. asperellumpool used in this study have the ability to acidify the rhizosphere region, which may explain the increase in availability and uptake of Zn for rice plants.
Application forms of beneficial microorganisms did not present an effect about the nutrient content in shoots of rice plants (Table 4). The only exception was Mn, in which application on the seeds and seed-plant provided similar results. Then, conclude that for provide higher Mn uptake and other nutrients, application form of beneficial microorganisms in the rice plants, by seed microbiolization, must be indicated, since that is a procedure less cost to farmers. Nascente et al. (2017a,b) also reported that to nutrients uptake, just seed microbiolization could be sufficient to achieve the beneficial effects promoted by microorganisms.
Rhizobacteria evaluated in the present work were collected on the rhizosphere of upland rice (Filippi et al., 2011). However, they promoted significant increases in the gas exchange and shoot biomass of lowland rice (Tables 2 and 3). In upland rice, interaction among BRS Primavera cultivar and isolates BRM32109, BRM32111, BRM32113, BRM32114 and T. asperellumalso provided increase on the root length (Rêgo et al., 2014; Sperandio et al., 2017) and, higher biomass production was provided in rice cultivar treated with isolate BRM32114 (Serratiasp.) (Nascente et al., 2017b). Besides, in lowland rice, BRS Catiana cultivar treated with BRM32109 (Bacillussp.) provided higher biomass production (Nascente et al., 2017a) and, in this present study, improvements in shoot biomass of tropical lowland rice, BRS A702 CL cultivar, was achieved with isolates BRM32113 (Burkholderia pyrrocinia) and BRM32109 (Table 3). Thus, it can be inferred that microorganisms could act differently in different cultivars of the same crop species, confirming reports made by Mendes et al. (2018).
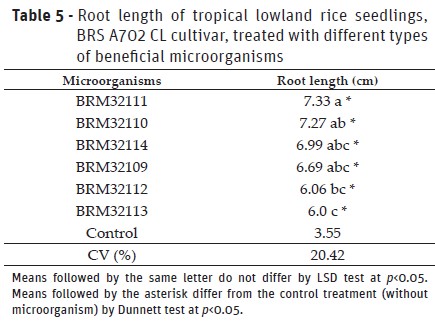
Experiment II: Effect of beneficial bacteria on the root system of tropical lowland rice seedlings
BRM32111 (P. fluorescens) provided the highest root growth on rice seedling and, differed to the BRM32113 (B. pyrrocinia) and BRM32112 (Pseudomonassp.) (Table 5). Additionally, the six isolates evaluated provided increases, on average, of 89% on root length of plants and differed to the control treatment. Studies with upland rice showed that seeds treated with isolates BRM32114 (Serratiasp.) and BRM32109 (Bacillussp.) (Sperandio et al., 2017), and T. asperellumpool, BRM32113 (B. pyrrocinia) and BRM32111 (P. fluorescens) (Rêgo et al., 2014) produced seedling with increased on the root length compared to the control treatment. Beneficial microorganisms can produce vegetable hormones like auxins, cytokinins, and gibberellins, which provided better development of the plant root structure (Oliveira et al., 2003). Higher root development on the plants by the use of beneficial microorganisms can provide increases on the water and minerals uptake, resulting in the plants more vigorous and productive (Hungria, 2011).
Thinking about to sustainability of systems production, these results of higher biomass production with plants treated with beneficial microorganisms, give real expectative of the use of beneficial microbial, in which are friendly environmental technologies, whose most important objective is the reduction of the use of the synthetic inputs as fertilizers and fungicides.
Besides of promising results, investigations with these microorganisms, BRM32113 and BRM32109 in BRS A702 CL cultivar should be extended to field conditions to prove the benefits of these interactions. Parallel, other investigations should be carried out, seeking out elucidate physiological and metabolic permanent alterations that occur inside the plants that provide better agronomic performance on crops.
CONCLUSIONS
Rhizobacteria provided increases in gas exchange rates of rice plants. Overall, the application of beneficial microorganisms in seed-plant was efficient to provide benefits provided by these microorganisms. The isolates of rhizobacteria tested promoted increases, on average, of 89% on root length of rice seedlings. Tropical lowland rice treated with isolates BRM32109 (Bacillus sp.) and BRM32113 (B. pyrrocinia) presented an increase, on average, of 19% in shoot dry matter.
References
Abreu, C.A.; Lopes, A.S. & Santos, G.C.G. (2007) - Micronutrientes. In: Novais, R.F.; Alvarez, V.V.H.; Barros, N.F.; Fontes, R.L.F.; Cantarulli, R.B. & Neves, J.C.L. (Eds.) - Fertilidade do solo. Viçosa, UFG, p. 645-736. [ Links ]
Ahemad, M. & Kibret, M. (2014) - Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science, vol. 26, n. 1, p. 1-20. https://doi.org/10.1016/j.jksus.2013.05.001 [ Links ]
Cuevas, V.C.; Sinohin, A.M. & Orajay, J.I. (2005) - Performance of selected Philippine species of Trichoderma as biocontrol agents of damping off pathogens and as growth enhancer of vegetables in farmer's field. Philippine Agricultural Scientist, vol. 88, n. 1, p. 63-71. [ Links ]
Donagema, G.K.; Campos, D.V.B.; Calderano, S.B.; Teixeira, W.G. & Viana, J.H.M. (2011) - Manual de métodos de análise de solo. 2ª ed. Rio de Janeiro, Embrapa Solos, 230 p. [ Links ]
Filippi, M.C.C.; Silva, G.B.; Lobo, V.L.S.; Cortes, M.M.C.B.; Moraes, A.J.G. & Prabhu, A.S. (2011) - Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biological Control, vol. 58, n. 2, p. 160-166. https://doi.org/10.1016/j.biocontrol.2011.04.016 [ Links ]
França, S.K.S.; Cardoso, A.F.; Lustosa, D.C.; Ramos, E.M.L.S.; Filippi, M.C.C. & Silva, G.B. (2015) - Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agronomy for Sustainable Development, vol. 35, n. 1, p. 317-324. https://doi.org/10.1007/s13593-014-0244-3 [ Links ]
Gálmes, J.; Ribas-Carbó, M.; Medrano, H. & Flexas, J. (2011) - Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2concentration under water stress. Journal of Experimental Botany, v. 62, n. 2, p. 653-665. https://doi:10.1093/jxb/erq303 [ Links ]
Gonçalves, G.M.O. (2016) - Atributos químicos do solo de várzea tropical cultivado com arroz irrigado em razão do manejo de nitrogênio. Dissertação de mestrado. Goiânia, Universidade Federal de Goiás. 62 p. [ Links ]
Hungria, M. (2011) - Inoculação com Azospirillum brasiliense: inovação em rendimento a baixo custo. 1ª ed. Londina, Embrapa Soja, 35 p. [ Links ]
Isawa, T.; Yasuda, M.; Awasaki, H.; Minamisawa, K.; Shinozaki, S. & Nakashita, H. (2010) - Azospirillum sp. strain B510 enhances rice growth and yield. Microbes and Environments, vol. 25, n. 1, p. 58-61. https://doi.org/10.1264/jsme2.ME09174 [ Links ]
Kado, C.J. & Heskett, M.G. (1970) - Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas and Xanthomonas. Phytopathology, vol. 60, n. 6, p. 969-976. https://doi.org/10.1094/Phyto-60-969 [ Links ]
Kumar, V. & Ladha, J.K. (2011) - Direct seeding of rice: recent developments and future research needs. Advances in Agronomy, vol. 111, p. 297-396. https://doi.org/10.1016/B978-0-12-387689-8.00001-1 [ Links ]
Malavolta, E.; Vitti, G.C. & Oliveira, S.A. (1997) - Avaliação do estado nutricional de plantas: princípios e aplicações. 2ª ed. Piracicaba, Potafós, 319 p. [ Links ]
Mattos, M.L.T.; Barrigossi, J.A.F. & Lanna, A.C. (2006) - Impacto da orizicultura na qualidade do meio ambiente. In: Santos, A.B.; Stone, L.F. & Vieira, N.R.A.A. (Eds.) - Cultura do Arroz no Brasil. Santo Antônio de Goiás, Embrapa Arroz e Feijão, p. 933-982. [ Links ]
Mendes, L.W.; Raaijmakers, J.M.; Hollander, M.; Mendes, R. & Tsai, S.M. (2018) - Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME Journal, vol. 12, n. 1, p. 212-224. https://doi.org/10.1038/ismej.2017.158 [ Links ]
Nascente, A.S.; Filippi, M.C.C.; Lanna, A.C.; Sousa, T.P.; Souza, A.C.A.; Lobo, V.L.S. & Silva, G.B. (2017a) - Effects of beneficial microorganisms on lowland rice development. Environmental Science and Pollution Research, vol. 24, n. 32, p. 25233-25242. https://doi.org/10.1007/s11356-017-0212-y [ Links ]
Nascente, A.S.; Filippi, M.C.C.; Lanna, A.C.; Souza, A.C.A.; Lobo, V.L.S. & Silva, G.B. (2017b) - Biomass, gas exchange, and nutrient contents in upland rice plants affected by application forms of microorganism growth promoters. Environmental Science and Pollution Research, vol. 24, n. 3, p. 2956-2965. https://doi.org/10.1007/s11356-016-8013-2 [ Links ]
Oliveira, A.L.M.; Urquiaga, S. & Baldani, J.I. (2003) - Processos e mecanismos envolvidos na influência de microrganismos sobre o crescimento vegetal. 1ª ed. Seropédica, Embrapa Agrobiologia, 40 p. [ Links ]
Qin, R.; Stamp, P. & Richner, W. (2005) - Impact of tillage and banded starter fertilizer on maize root growth in the top 25 centimeters of the soil. Agronomy Journal, vol. 97, n. 3, p. 674-683. https://doi.org/10.2134/agronj2004.0059 [ Links ]
Rêgo, M.C.F.; Ilkiu-borges, F.; Filippi, M.C.C.; Gonçalves, L.A. & Silva, G.B. (2014) - Morphoanatomical and Biochemical Changes in the Roots of Rice Plants Induced by Plant Growth-Promoting Microorganisms. Journal of Botany, vol. 2014, art. 818797. http://dx.doi.org/10.1155/2014/818797 [ Links ]
Santos, H.G. Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo-filho, J.C.; Oliveira, J.B. & Cunha, T.J.F. (2018) - Sistema brasileiro de classificação de solos. 5ª ed. Rio de Janeiro, Centro Nacional de Pesquisa de Solos, 356 p. [ Links ]
Shakeel, M.; Rais, A.; Hassan, M.N. & Hafeez, F.Y. (2015) - Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Frontiers in Microbiology, vol. 6, art. 1286. http://doi.org/10.3389/fmicb.2015.01286 [ Links ]
Silva, M.A.; Jifon, J.L.; Santos, C.M.; Jadoski, C.J. & Silva, A.G. (2013) - Photosynthetic Capacity and Water Use Efficiency in Sugarcane Genotypes Subject to Water Deficit During Early Growth Phase. Brazilian Archive of Biology and Technology, vol. 56, n. 5, p. 735-748. http://doi.org/10.1590/s1516-89132013000500004 [ Links ]
Silva, J.C.; Torres, D.B.; Lustosa, D.C.; Filippi, M.C.C. & Silva, G.B. (2012) – Rice sheath blight biocontrol and growth promotion by Trichodemraisolates from the Amazon. Amazonia Journal of Agricultural and Environmental Sciences, vol. 55, n. 4, p. 243-250. http://dx.doi.org/10.4322/rca.2012.078 [ Links ]
Silva, V.N.; Guzzo, S.D.; Lucon, C.m.m. & Harakava, R. (2011) - Promoção de crescimento e indução de resistência à antracnose por Trichoderma spp. em pepineiro. Pesquisa Agropecuária Brasileira, vol. 46, n. 12, p. 1609-1817. http://doi.org/10.1590/s0100-204x2011001200005 [ Links ]
Sousa, T.P.; Souza, A.C.A.; Filippi, M.C.C.; Lanna, A.C.; Cortês, M.V.; Pinheiro, H.A. & Silva, G.B. (2018) - Bioagents and silicon promoting fast early upland Rice growth. Environmental Science and Pollution Research, vol. 25, n. 4, p. 3657-3668. http://doi.org/10.1007/s11356-017-0753-0 [ Links ]
Sperandio, E.M.; Vale, H.M.M.; Reis, M.S.; Cortes, M.V.C.B.; Lanna, A.C. & Filippi, M.C.C. (2017) - Evaluation of rhizobacteria in uplant rice in Brazil: growth promotion and interaction of induced defense responses against leaf blast (Magnaporthe oryzae). Acta Physiologiae Plantarum, vol. 39, n. 1, p. 258-270. http://doi.org/10.1007/s11738-017-2547-x [ Links ]
United Nations (2017) -World Population Prospects: The 2017 revision, Key findings and advance tables.Department of Economic and Social Affairs. 46 p. [ Links ]
Received/recebido: 2019.05.15
Accepted/aceite: 2019.07.19