Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. vol.28 no.1 Dois Portos jun. 2013
Comparative leaf micromorphoanatomy of Vitis vinifera ssp. vinifera (Vitaceae) red cultivars
Micromorfoanatomia foliar de cultivares tintas de Vitis vinifera ssp. vinifera (Vitaceae)
Ana Monteiro*1, Generosa Teixeira2, Carlos M Lopes1
1Centro de Botânica Aplicada à Agricultura, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Tapada da Ajuda, 1349-017 Lisbon, Portugal, e-mail: anamonteiro@isa.utl.pt; carlosmlopes@isa.utl.pt
2Universidade de Lisboa, Faculdade de Farmácia, Centro de Biologia Ambiental, Av. Prof. Gama Pinto. 1649-003 Lisbon, Portugal, e-mail: gteixeira@ff.ul.pt
*Corresponding author:
SUMMARY
Aiming to characterize and discriminate between four red grapevine cultivars – Aragonez (AR), Cabernet Sauvignon (CS), Syrah (SY) and Touriga Nacional (TN) – grown under Mediterranean field conditions, we studied their leaf micromorphoanatomic characteristics under light (LM) and scanning electron microscopy (SEM). The studied characteristics included those of the epidermis, stomata and hair distribution, and the mesophyll structure. The individual primary leaf area revealed significant differences between cultivars, with the highest value presented by AR and the lowest by CS, while SY and TN gave intermediate values. CS presented a significantly higher leaf specific dry weight value than the other three cultivars, which returned similar values. Under SEM magnification three types of stomata were identified in all the studied genotypes: sunken, at the same level, and raised above the other epidermal cells. Each cultivar displayed different percentages of these types of stomata: the highest raised-above values were observed in AR; TN had the highest same-level values and the lowest sunken ones; CS revealed the highest values for sunken stomata; while SY returned average values for all the types of stomata. Stomatal density was higher in AR and SY and lower in CS and TN. The hairs on the lower surface presented a similar woolly aspect in all the studied cultivars, but the mesophyll structure was quite different: CS presented the highest and AR the lowest values for total thickness of the lamina, thickness of palisade and spongy parenchyma, and length and thickness of upper and lower epidermal cells; the values for these leaf features in TN and SY fell between those for CS and AR. The data suggest that differences in leaf micromorphoanatomy can be used to distinguish between grapevine cultivars. Further studies are needed to confirm whether there is any association between some of these leaf traits – e.g. stomata type and mesophyll structure – and the physiological behaviour observed under field conditions.
Key words: grapevine, leaf, epidermis, stomata, mesophyll.
RESUMO
Este estudo teve por objetivo caraterizar e comparar as características micromorfoanatómicas foliares de quatro cultivares tintas de videira – Aragonez (AR), Cabernet Sauvignon (CS), Syrah (SY) e Touriga Nacional (TN) – cultivadas em condições de campo com clima mediterrânico, com recurso à microscopia ótica (LM) e eletrónica de varrimento (SEM). Observaram-se os seguintes carateres foliares: epiderme superior e inferior, estomas, indumento e estrutura do mesófilo. A área foliar da folha principal apresentou diferenças significativas entre cultivares, apresentando AR o maior valor e CS o menor e SY e TN valores intermédios. CS apresentou uma área foliar específica significativamente superior ao das outras cultivares. Por recurso à microscopia eletrónica, identificaram-se três tipos de estomas nas quatro cultivares – enterrados, ao mesmo nível e elevados em relação às células epidérmicas. A proporção de cada tipo de estoma variou significativamente entre cultivares: AR apresentou uma maior percentagem de estomas elevados; TN os maiores valores de estomas ao mesmo nível e os menores dos estomas enterrados; CS os maiores valores de estomas enterrados; enquanto SY apresentou percentagens intermédias dos três tipos de estomas. A densidade estomática foi maior em AR e SY e menor em CS e TN. O indumento da página inferior foi semelhante nas quatro cultivares. A estrutura do mesófilo apresentou diferenças significativas entre as quatro cultivares. Os maiores e menores valores da espessura total do limbo, do parênquima clorofilino em paliçada e lacunoso e do comprimento e espessura das células da epiderme superior e inferior foram apresentados pelo CS e AR, respetivamente, enquanto que a TN e a SY apresentaram valores intermédios destes carateres. Os resultados mostram diferenças significativas na micromorfoanatomia foliar dos genótipos estudados sublinhando a necessidade de mais estudos para averiguar possíveis interações entre carateres anatómicos foliares – tipo de estomas e estrutura do mesófilo, por exemplo – e respostas fisiológicas observadas em condições de campo.
Palavras-chave: videira, folha, epiderme, estomas, mesófilo.
INTRODUCTION
It is well known that plants have developed functional and structural features that allow them to survive in unfavourable environments. This diversity of structure is as great as taxa biodiversity. Grapevine (Vitis vinifera L. ssp. vinifera) leaves are a good example, as they exhibit several adaptive mechanisms – a reduction in leaf size, or an increase in mesophyll compactness (Esau, 1977; Dickison, 2000), for instance. Under heat and water stress conditions plants decrease the size of their epidermal cells, increase epidermal cell density and thickness, and stomata became more numerous and smaller (Gokbayrak et al., 2008; Salem Fnayou et al., 2010). Grapevine cultivars have also been reported to adapt to water deficit, mainly by modifying their morphological and anatomical characteristics (Gómez-del-Campo et al., 2003, 2004; Koundouras et al., 2008; Costa et al., 2012). Descriptive botanical works on the ampelography (Galet, 2000), morphology and anatomy (Pratt, 1974; Esau, 1977; Metcalfe and Chalk, 1979) of grapevines were published in the early twentieth century. More recently, grapevine cultivars have been characterised by ampelographic descriptors based on morphology and morphometry (Mota, 1986; Dettweiler, 1991; Coelho et al., 2004), and in some cases, on microsatellite markers (Santiago et al., 2005; Lopes et al., 2006; Veloso et al., 2010). Grapevine cultivar susceptibility and resistance to downy mildew associated with differences in leaf morphology at the macro- and microscopic levels were studied by Boso et al. (2010, 2011). Few attempts have been made to employ leaf micromorphology traits to explain heat and water stress tolerance (Gómez-del-Campo et al., 2003; Koundouras et al., 2008; Costa et al., 2012). Recently, Sadras et al. (2012) reported that stomata density of the Shiraz cultivar was unaffected by temperature, but stomata length and width increased with heat. They concluded that longer and wider stomata contributed to the enhanced plasticity of stomatal conductance under higher temperatures.
Micromorphological grapevine leaf traits, such as stomata (Denisov, 1970; Sievers, 1971; Hegedüs, 1974; Pratt, 1974; Düring, 1980; Boso et al., 2011), trichomes (Hegedüs, 1974; Pratt, 1974), mesophyll (Pratt, 1974; Patakas et al., 2003) and leaf surfaces (Salem-Fnayou et al., 2010; Boso et al., 2011), have been studied. Differences have been observed between V. vinifera cultivars in leaf morphoanatomical features, although few works have used them to classify the studied cultivars (Swanepoel and Villers, 1987; Boso et al., 2011). The latter researchers concluded that the most important micromorphological characteristics for distinguishing between genotypes are stomatal frequency and trichome distribution.
According to the review by Pratt (1974), grapevine mainly has stomata on its lower epidermis, which may be raised above, at the same level and sunken, relatively to the surface of the epidermis. Swanepoel and Villers (1987) also described all three types of stomata in the genus Vitis. Boso et al. (2011) reported that the stomata of the cv. Blanco Legitimo stood out in that it displayed a type of doming of the epidermis – a feature that was not seen in any other of the genotypes they studied.
According to Schanderl (1968) and Pratt (1974), in grapevine the trichomes appear especially on the lower surface of the leaf and are uni- or pluricellular, dead and woolly, or living and woolly, round or flat, or twisted if long. Boso et al. (2011) observed reclining and erect hairs under the scanning microscope at different magnifications. The reclining hairs appeared as filaments of different sizes, while the erect hairs took the shape of small spikes. Patakas et al. (2003) and Boso et al. (2011) observed that some genotypes presented differences in palisade and spongy thickness and in exposed upper epidermal cell area.
The knowledge of the structure of epidermis and mesophyll cells is meaningful in terms of environmental species adaptation. The Mediterranean climate experiences periods of high light and drought that greatly influence the plant growth and development, yield and the quality attributes of the grapes. Some differences among grapevine cultivars have been observed with regard to their ability to adapt and produce under drought conditions. For example, under the same Mediterranean field growth conditions the red grapevine cultivars Aragonez, Cabernet Sauvignon, Syrah and Touriga Nacional diverge in their responses to heat and drought in summer conditions (mid-ripening – late August), with Cabernet Sauvignon' and Touriga Nacional being more tolerant than Aragonez' (Costa et al., 2012).
In the present study leaf micromorphoanatomic-biometric characteristics of the V. vinifera ssp. vinifera red cultivars Aragonez, Cabernet Sauvignon, Syrah and Touriga Nacional were analysed at the light and scanning electron microscope level, with the goal of distinguishing between the leaf anatomy of the genotypes.
MATERIAL AND METHODS
Plant material and growth conditions
Five-year-old field-grown grapevines of the red cultivars Aragonez (AR), Cabernet Sauvignon (CS), Syrah (SY) and Touriga Nacional (TN) grafted on SO4 rootstocks were studied at a non-irrigated vineyard located near Torres Vedras, 60 km north of Lisbon, within the Lisbon Winegrowing Region (39º 01 N, 9° 06 E). The soil is a sandy clay loam with 1.45% organic matter and pH 5.9. The vineyard has a planting density of 4,000 vines per hectare, spaced 1.0 m within and 2.5 m between north-south oriented rows. Vines were trained on a vertical shoot positioning with two pairs of movable wires, and spur-pruned on a bilateral Royat Cordon system. All vines were uniformly pruned to 14-16 nodes per vine. Shoots were trimmed twice, between bloom and veraison, at a height of about 1.5 m.
The vineyard is a small varietal collection, where the four studied cultivars are planted side by side, with three adjacent rows of 60 plants per cultivar under the same canopy and cultivation management. For data collection, four blocks of 10 vines per cultivar were established along the central row.
Morphoanatomical analysis
In 2007, six full-expanded leaves per block from each cultivar were taken from the 8th node of the shoot, at veraison (end of July). Five small leaf blade sections were cut from the central leaf part, between the main and lateral veins. All measurements and counts related with morphoanatomical traits were done on random fields under light (LM) and scanning electron microscopy (SEM), always at comparable leaf situations and magnifications.
Light microscopy (LM)
Fresh material and dried material were placed in sodium hypochlorite until bleached white, and then washed in distilled water. These LM observations focused on the upper and lower epidermal characteristics, such as epidermal cell shape.
At the same time, pieces of fresh leaves of each cultivar were fixed with 2.5 % glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.0 (Hayat, 1981). Samples were then washed and tissue dehydration was carried out in an ascendant alcohol series and processed using the paraffin micro-technique (Ruzin, 1999). The blocks were sectioned (10-12 μm) in a Minot microtome and examined with a Nikon Labophot 2 photomicroscope. Images were obtained with a Nikon FX-35W camera equipped with a semi-automatic Nikon PFX adapter (Nikon®). These LM observations focused on the transverse sections and addressed total lamina thickness, thickness of palisade and spongy tissues, upper cuticle thickness, length of upper and lower epidermal cells, and thickness of upper and lower epidermal cells.
Scanning electron microscopy (SEM)
Plant material was fixed as above, critical-point dried in a Critical Point Polaron BioRad E3500, and coated with gold in a Jeol JFC-1200. Observations were carried out at 15 kV on a Jeol JSM-5220 LV scanning electron microscope equipped with a direct image acquisition system. Measurements and counts were obtained by computer-assisted image analysis.
SEM observations focused on the upper and lower epidermis surface details – type of indumentum, epicuticular waxes, stomata density (stoma mm-2), type of stomata and percentage of total of each type, and stomata cell length and width. The type of stomata followed the nomenclature given by Pratt (1974), who considered three types: rose above, at the same level, and sunken to the level of the other epidermal cells.
Vegetative growth
Individual primary leaf area was estimated using the methodologies proposed by Lopes and Pinto (2000). Leaf dry weight and specific dry weight were assessed at veraison in a random sample of 12 leaves (8th node).
Data analysis
Anatomical data were analyzed by one-way ANOVA in accordance with GLM procedures from the SAS® program package (SAS Institute, Cary, NC, USA), and statistical differences between means were assessed by LSD test (α < 0.05). Stomata type (% of total) was analysed in terms of non-transformed and transformed (arcsine of the square root) values. As arcsine transformation did not alter the interpretation of the data, thus non-transformed mean figures for stomata type are presented.
RESULTS
Micromorphoanatomical traits
The leaf morphoanatomic-biometric characteristics of the studied V. vinifera cultivars are summarised in Tables I and II. The individual primary leaf area of the 8th leaf reveals significant differences between cultivars, with the highest value presented by AR and the lowest by CS, while SY and TN gave intermediate values. CS presented a significantly higher leaf specific dry weight value than the other three cultivars, which obtained similar values (Table I).
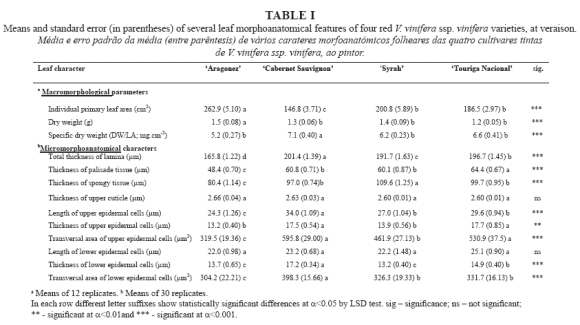
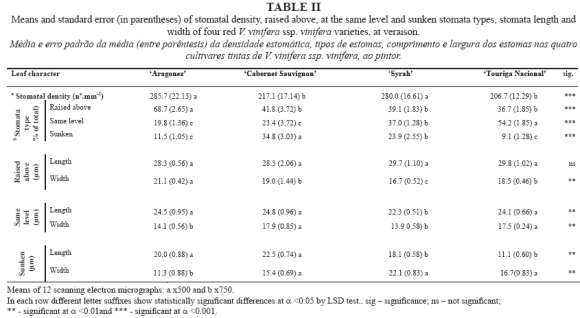
The epidermal cells of the upper leaf surface were uniform, rectangular or slightly polygonal in shape, with straight walls and some striate in the cuticle surface (Fig. 1). In terms of the length of the upper epidermal cells CS showed the significantly highest values and AR the lowest, while both SY and TN gave intermediate values (Table I).
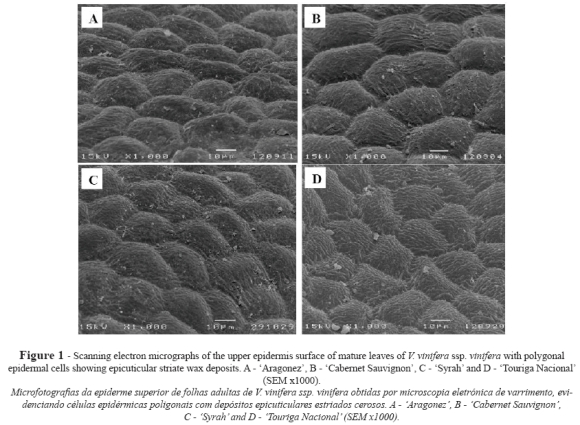
In all the cultivars the lower epidermis revealed rectangular or slightly polygonal cells with sinuous cell walls and similar length (Fig. 2, Table I). Cuticular striations were seen in all cultivars, but were scarcer in CS (Fig. 2B).
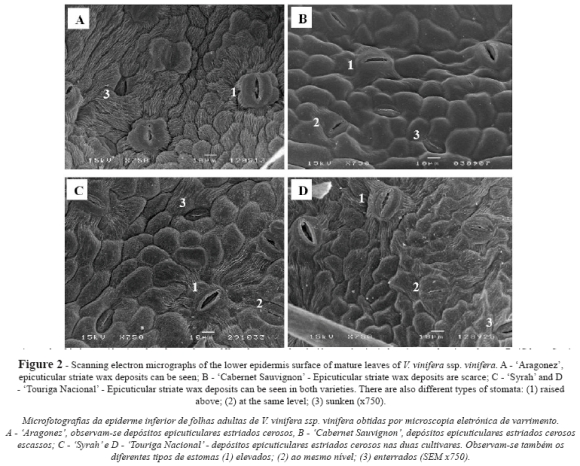
Stomata density was determined from SEM images, with a significantly higher value in AR and SY compared to CS and TN (Table II). Three types of stomata coexisted in the lower epidermis of all cultivars (Fig. 2): raised above (the guard cells are above, and each flanked by curved subsidiary cells), same level (the guard cells are flattened with the subsidiary cells) and sunken (the guard cells are buried in the subsidiary cells). The prevalence of one type over the others varied significantly with genotype (Table II): AR showed a significantly higher percentage of raised-above stomata than the other three cultivars, which presented statistically similar values. In terms of the proportion of same-level stomata, TN produced the highest values and AR and CS the lowest, while the SY values were intermediate. CS presented the highest percentage of sunken stomata, TN and AR the lowest, and SY intermediate values.
All types of stomata presented a random distribution and an irregular orientation, with size generally but not always decreasing with deepness level (Table II and Fig. 2). The raised-above type stomata exhibited a similar length in all cultivars, but significant differences in width. SY presented the smallest and AR the greatest width, differing significantly from that of TN and CS, which returned similar values (Table II). For the same-level type stomata, SY presented a significantly shorter stomata length than the other three cultivars, which presented statistically similar values; however, the width was significantly lower for AR and SY, compared to that of CS and TN. Regarding to the sunken type stomata, SY again presented significantly short stomata length than the other three cultivars, which presented statistically similar values. The width of the sunken type was significantly smaller in AR and SY, compared to that of CS and TN (Table II).
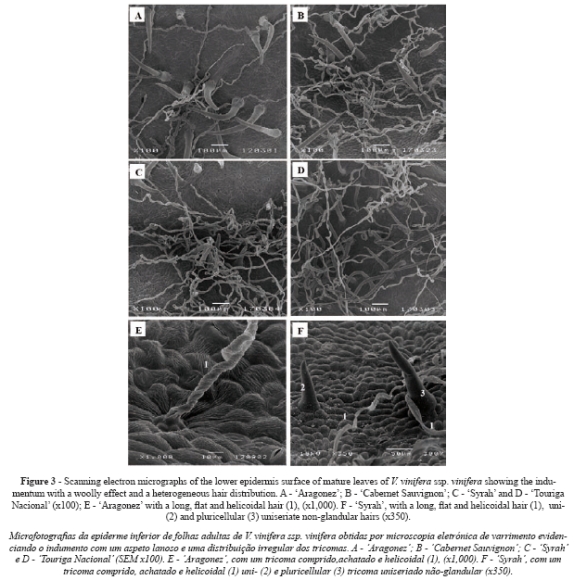
In all cultivars only the lower epidermis surface was pubescent (Fig. 3A, B, C and D). All cultivars presented two types of trichome on the main veins and over different areas of the limbus: i) uni- and pluricellular uniseriate non-glandular hairs, erect or slightly curved (Fig. 3A and 3F); ii) very long hairs, probably unicellular and usually flat with a helicoidal rolling (Fig. 3E), producing a network with a fluffy effect. Some of these long hairs are dead because they do not retain their protoplasts. All the kinds of hairs presented an irregular distribution, no orientation and a variable density within each cultivar (Fig. 3A, B, C and D). These features and the fluffy long hairs make it impossible to assess trichome density.
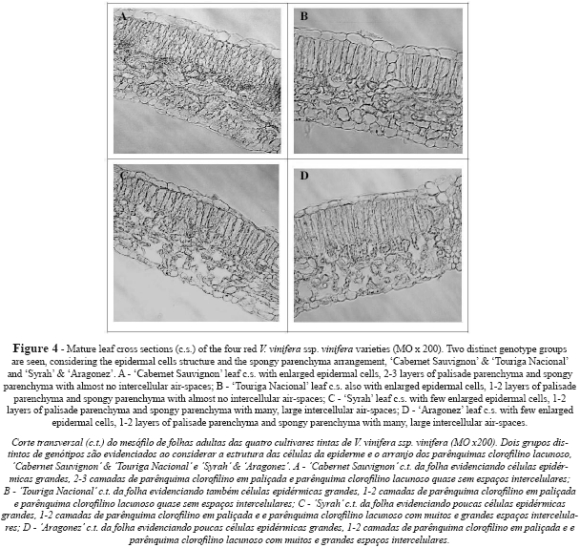
Leaf cross sections revealed dorsiventral leaves with a single layer of protection tissue. AR and SY presented a tendency towards flattened epidermal cells, while CS and TN tended to have almost circular cells in both the upper and the lower epidermis (Table I and Figures 4 and 5A). The upper cuticle thickness was similar in all cultivars. Palisade parenchyma displayed compact cells arranged in 1-2 layers in all cultivars (Fig. 4), but in CS three layers were also observed in some transverse sections. Despite this, the thickness of the palisade parenchyma was greater in TN, intermediate in CS and SY, and smaller in AR (Table I). The spongy parenchyma cells were much more heterogeneous in shape and were partly separated from one another by irregular intercellular air-spaces, which were larger and more clearly visible on the leaves of SY (Fig. 4C) and AR (Fig. 4D), compared to those of CS (Fig. 4A) and TN (Fig. 4B and 5A). The thickness of spongy tissue was greater in SY, intermediate in CS and TN, and smaller in AR (Table I). Total thickness of the lamina showed significant differences between all cultivars (Table I), with CS being the cultivar with the thickest leaf lamina, followed by TN, SY and AR. These anatomic features are in accordance with the specific dry weight values, as CS has the smallest and thickest leaf and AR the largest and thinnest one, while SY and TN presented intermediate values (Table I).

During the observation of cross sections, enlarged idioblasts were visible, widely distributed in the parenchyma tissues, with two different types of calcium oxalate crystals inside: raphides (Fig. 5B) and druses.
DISCUSSION
All four genotypes displayed upper and lower unistratified epidermal cells with thin walls and a thin cuticle. They had almost the same rectangular or slightly polygonal cell shape – features similar to those reported by Galet (2000) and Boso et al. (2010).
In CS, values for transverse epidermal cell surface area and epidermal thickness were higher than those obtained by Boso et al. (2010, 2011) in Spain, while SY epidermal thickness values were lower than the ones reported by Patakas et al. (2003) in Greece. Under our conditions, TN presented transverse epidermal cell surface values similar to those for SY, but AR presented the lowest ones. These differences seem to indicate that ecological conditions may influence grapevine anatomical features. Some authors have pointed out that larger cellular lumen might be involved in water storage, which may have some physiological significance (Esau, 1977; Dickison, 2000). Salem-Fnayou et al. (2010) observed that under heat stress the grapevine cultivars Razegui and Muscat Italia exhibited elongated convex epidermal cells with a less sinuous shape than those of control leaves that were not submitted to heat stress.
Martin and Juniper (1970) stated that the study of cuticles provides important information about plant habitat and its response to abiotic stresses. In our study, cuticular striations were seen on both upper and lower surfaces, especially around the stomata. Wax deposits were very few or scarce – e.g. in CS – with Boso et al. (2011) reporting a similar observation.
Average cuticular thickness for all the cultivars was 2.6 μm, a value that is higher than the one Salem-Fnayou et al. (2010) reported for the table grape cultivars Muscat Italia and Razegui. These differences might be determined by the genotype and the growth conditions – namely drought and heat stress (Wahid et al., 2007). Indeed, under heat stress conditions Salem-Fnayou et al. (2010) reported a significant decrease in cuticle thickness in their cultivars, followed by an increase in epidermal cell wall thickness.
Stomata classification varies from one author to another, making comparison between studies difficult. In a review published by Pratt (1974), three types of stomata – raised above, same level, and sunken – were identified in the genus Vitis. This classification is not easy, inasmuch as it was only with magnifications of more than 750x that it was possible to identify these three types of stomata. Indeed, their small size and position relative to the other epidermal cells meant that sunken stomata were not clearly distinguished with lower magnifications, with implications for stomatal densities, for example. In our study, with LM microscopy it was impossible to apply the Pratt classification and distinguish between the three types of stomata.
All the genotypes exhibited the three types of stomata, but the proportion of each type varied with genotype. Swanepoel and Villers (1987) also observed that stomata on the same leaf could be raised, same level and sunken; however, they did not present any data on the relative proportion of each type. Working at electron microscope level on the variability in leaves with 11 Vitis vinifera and three non-vinifera genotypes, Boso et al. (2011) stated that the stomata of the Blanco Legítimo cultivar stood out because it displayed a type of doming of the epidermis that corresponds to the raised-above type. However, they did not see this type of stomata on the other studied genotypes.
In our study, stomata density varied significantly with genotype, with values of the same order of magnitude as those presented for several other Vitis genotypes by Swanepoel and Villers (1987). Having said this, for the same grapevine cultivars we found higher stomatal densities than the ones reported by other authors (Boso et al., 2010, 2011; Costa et al., 2012). One explanation for the observed differences may be the method we used for stomata counting and differentiation with a 750x scanning electron micrograph. When we compared the counts obtained by LM with the SEM results, we realized that neither the sunken, nor the raised-above stomata were visualized by LM. This methodological limitation could be the main reason why the literature reports stomatal densities below our data. Salem-Fnayou et al. (2010) observed no significant differences in stomata densities between heat-stressed and control grapevines, and concluded that grapevine plants maintained fairly constant stomata density in order to avoid excess transpiration due to heat stress, and that stomata density is affected more by drought than by heat stress.
In addition, within each type of stomata significant differences between the four cultivars were found in stomata length and width. Boso et al. (2011) reported the same results, and concluded that there was no clear relationship between leaf blade area and stomata size.
Our study showed that when the stomatal index was determined with LM, it presented no significant differences between the genotypes. These results can be explained by the low tissue amplification (the deepest stomata were probably just not seen) and also by the inherent difficulty of distinguishing the epidermal cells from one another, thus affecting the ability to count them. Wilkinson (1979) and Stace (1989) stated that it is better to record the most frequent size or the length-to-width ratio of the stomata, rather than the stomatal index.
Regarding the indumentum found in the lower epidermis, our results pointed to the presence of uni- and pluricellular uniseriate non-glandular hairs, which were erect or slightly curved, and very long hairs, which were usually flat with helicoidal rolling. The pattern of hair distribution is either absent or essentially the same in all the cultivars. Working on six grapevine genotypes, including CS, Boso et al. (2010) were the first to mention the same types of trichome in Vitis leaves. The influence of the indumentum on the development of plant organs and its latters role in several aspects of physiological and ecological adaptation are well known (Theobald et al., 1979).
Mesophyll organization and thickness presented significant differences between cultivars. CS and TN displayed compact cells in both palisade and spongy parenchyma, while SY and AR presented an opposite pattern, inasmuch as their spongy parenchyma exhibited air spaces that were both numerous and large. The same differences were also observed between other cultivars by Salem-Fnayou et al. (2010) and Boso et al. (2010). It is known that this mesophyll arrangement may be related with downy mildew resistance (Gindro et al., 2003; Boso et al., 2010) and leaf gas exchanges (Evans et al., 1994; Syvertsen et al., 1995; Patakas et al., 2003).
In our study we noticed the coexistence of raphids and druses in all the samples, at veraison. Calcium oxalate crystals are a major bio-mineralization product that is typically developed within specialized cells called idioblasts, but their biological function and plant use are still not properly understood (Prychid and Rudall, 1999; Jáuregui-Zúñiga et al., 2003; Nakata, 2003).
CONCLUSIONS
Our data showed that many leaf micromorphoanatomical features of the grapevine cultivars Aragonez, Cabernet Sauvignon, Syrah and Touriga Nacional differ significantly. The leaf traits that contributed most to distinguishing between grapevine cultivars were: stomata density, type of stomata (%), size of stomata cells, and mesophyll characteristics.
Discrepancies in stomata type and density recorded in the literature for the genus Vitis indicate that in future works, methodological standardization – e.g. of sample preparation and microscope magnification – is recommended in order to clarify this important anatomical feature.
Further studies are needed to confirm whether there is any association between some of these leaf traits – stomata type and mesophyll structure, for example – and the physiological behaviour observed under field conditions.
ACKNOWLEDGMENTS
We would like to thank Telmo Nunes, Faculdade de Ciências da Universidade de Lisboa, for his technical assistance with SEM.
REFERENCES
Boso S., Allonso-Villaverde V., Santiago J.L., Gago P., Durenberber M., Duggelin M., 2010. Macro and microscopic leaf characteristics of six grapevine genotypes (Vitis spp.) with different susceptibilities to grapevine downy mildew. Vitis, 49, 43-50. [ Links ]
Boso S., Gago P., Alonso-Villaverde V., Santiago J.J., Mendez J., Pazos I., Martínez M.C., 2011. Variability at the electron microscopy level in leaves of members of the genus Vitis. Sci. Horticulturae, 128, 228-238. [ Links ]
Coelho I., Cunha J., Cunha J.P., Carneiro L.C., Castro R., Eiras Dias J.E., 2004. Ampelometric comparison of wild vine Vitis vinifera L. populations and old grapevine cultivars of the South of Portugal. Ciência Téc. Vitiv., 19, 1-12 [ Links ]
Costa J.M., Ortuño M.F., Lopes C.M., Chaves M.M., 2012. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol., 39, 179–189. [ Links ]
Denisov N.I., 1970. Anatomical and morphological study of Vitis amurensis. Sb. Tr. Asp., 17, 401-408. [ Links ]
Dettweiler E., 1991. Preliminary Minimal Descriptor List of Grapevine Varieties. Institut für Rebenzüchtung Geilweilerhof, Siebeldingen. [ Links ]
Dickison W.C., 2000. Integrative Plant Anatomy. Harcourt Academic Press, San Diego. [ Links ]
Düring H., 1980. Stomatafrequenz bei Blättern von Vitis-species und Sorten. Vitis, 19, 91-98. [ Links ]
Esau K., 1977. Anatomy of seed plants. 2nd ed. John Wiley & Sons Inc, New York. [ Links ]
Evans J.R., Von Caemmerer S., Setchell B.A., Hudson G.S., 1994. The relationship between CO2 transfer and leaf anatomy in transgenic tobacco with a reduced content of rubisco. Aust. J. Plant Physiol., 21, 475–495. [ Links ]
Galet P., 2000. Précis de viticulture. 7éme. 602 p. Montpellier. [ Links ]
Gindro K., Pezet R., Viret O., 2003. Histological study of the responses of two Vitis vinifera (resistant and susceptible) to Plasmopara viticola infections. Plant Physiol. Biochem., 41, 846-853. [ Links ]
Gómez-del-Campo, M., Baeza P., Ruiz C., Lissarrague J.R., 2004. Water-stress induced physiological changes in leaves in four container-grown grapevine cultivars (Vitis vinifera L.). Vitis, 43, 99-105. [ Links ]
Gómez-del-Campo M., Ruiz C., Baeza P., Lissarrague J.R., 2003. Drought adaptation strategies of four grapevine cultivars (Vitis vinifera L.): modification of the properties of the leaf area. J. Int. Sci. Vigne Vin, 37, 131–143. [ Links ]
Gokbayrak Z., Dardeniz A., Bal M., 2008. Stomatal density adaptation of grapevine to windy conditions. Trak. J. Sci., 6, 18-22. [ Links ]
Hayat M., 1981. Principles and techniques of electron microscopy. Biological applications. 2nd ed. Arnold Publ., London. [ Links ]
Hegedüs A., 1974. Study of the epidermis of vine leaves. Acta Bot. Acad. Sci. Hung. 20, 225-270. [ Links ]
Jáuregui-Zúñiga D., Reyes-Grajeda J.P., Sepúlveda-Sánchez J.D., Whitaker J.R., Morenol A., 2003. Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifera. J. Plant Physiol., 160, 239–245. [ Links ]
Koundouras S., Tsialtas I.T., Zioziou E., Nikolaou N., 2008. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet-Sauvignon) under contrasting water status: leaf physiological and structural responses. Agr. Ecosyst. Environ., 128, 86-96. [ Links ]
Lopes C.M., Pinto P.A., 2000. Estimation de la surface foliaire principale et secondaire dun sarment de vigne. Prog. Agric. Vitic., 117, 160-166. [ Links ]
Lopes M.S., Rodrigues dos Santos M., Eiras Dias J.E., Mendonça D., Câmara Machado A., 2006. Discrimination of Portuguese grapevines based on microsatellite markers. J. Biotecnol., 127, 34-44. [ Links ]
Martin J.T., Juniper B.E., 1970. The cuticles of plants. Arnold Publ., London. [ Links ]
Metcalfe C., Chalk. L., 1979. Anatomy of Dicotyledons. Vol I. 2nd ed. Oxford Univ. Press. [ Links ]
Mota M.T. da Silva, 1986. Catálogo das Castas. Região Demarcada dos Vinhos Verdes. Ministério da Agricultura, Pescas e Alimentação. Instituto de Gestão e Estruturação Fundiária, Lisboa. [ Links ]
Nakata P., 2003. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci., 164, 901-909. [ Links ]
Patakas A., Kofidis G., Bosabalidis A M., 2003. The relationships between CO2 transfer mesophyll resistance and photosynthetic efficiency in grapevine cultivars. Sc. Horticulturae, 97, 255–263. [ Links ]
Pratt C., 1974, Vegetative anatomy of cultivated grapes – a review. Am. J. Enol. Vitic., 25, 131-150. [ Links ]
Prychid C., Rudall P., 1999. Calcium oxalate crystals in Monocotyledons: A review of their structure and systematics. Ann. Bot., 84, 725-739. [ Links ]
Ruzin S., 1999. Plant microtechnique and microscopy. Oxford University Press. [ Links ]
Sadras V.O., Montorob A., Morana M.A., Aphaloc P.J., 2012. Elevated temperature altered the reaction norms of stomatal conductance in field-grown grapevine. Agr. Forest Meteorol., 165, 35–42. [ Links ]
Salem-Fnayou A.B., Bouamama B., Ghorbel A., Mliki A., 2010. Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress. Microsc. Res. Techniq., 74, 756-762. [ Links ]
Santiago J.L., Boso S., Martín J.P., Ortiz J.M., Marínez M.C., 2005. Characterisation and identification of grapevine cultivars (Vitis vinifera L.) from northwestern Spain using microsatellite markers and ampelometric methods. Vitis, 44, 67-72. [ Links ]
Schanderl H., 1968. Uber die lebenden Haare auf den Blattadern von Reben. Wein-Wissenschaft, 23, 157-173. [ Links ]
Sievers E., 1971. Anatomische Unterscheide bei Klonen der Weisser Riesling. Mitt. Klosterneuburg, 21, 63-64. [ Links ]
Stace, C. 1989. Plant taxonomy and biosystematics. 2nd ed. Arnold Publ.. Ltd., London. [ Links ]
Syvertsen J.R., Lloyd J., Mc Conchie C., Kriedemann P.E., Farquhar G.D., 1995. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves, Plant Cell Environ., 18, 149–157. [ Links ]
Swanepoel J.J., Villiers C.E., 1987. A numerical-taxonomic classification of Vitis spp. and cultivars based on leaf characteristics. S. Afr. J. Enol. Vitic., 8, 31-35. [ Links ]
Theobald W., Krauhulik J., Rollins R., 1979. Trichome description and classification. In: Anatomy of Dicotyledons. 40-53. Metcalfe C., Chalk L. (eds.), Vol. I. 2nd ed. Oxford Univ. Press. [ Links ]
Veloso M.M., Almandanim M.C., Baleiras-Couto M., Pereira H.S., Carneiro L.C., Fevereiro P., Eiras-Dias J., 2010. Microsatellite database of grapevine (Vitis vinifera L.) cultivars used for wine production in Portugal. Ciência Téc. Vitiv., 25, 53-61. [ Links ]
Wahid A., Gelani S., Ashraf M., Fooland M.R., 2007. Heat tolerance in plants: An overview. Environment Exp. Bot., 61, 199-223. [ Links ]
Wilkinson H., 1979. The plant surface. In: Anatomy of Dicotyledons. 97-165. Metcalfe C., Chalk L. (eds.), Vol. I. 2nd ed. Oxford Univ. Press. [ Links ]
*Corresponding author:
Centro de Botânica Aplicada à Agricultura, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Tapada da Ajuda, 1349-017 Lisbon, Portugal, Fax: 213653195, e-mail: anamonteiro@isa.utl.pt
(Manuscrito recebido em 07.05.2013. Aceite para publicação em 09.07.2013)













