Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. vol.26 no.1 Dois Portos 2011
Commercial Sanitizers Efficacy – A Winery Trial
Filomena L. Duarte1*, Alberto López1, M. Filomena Alemão1, Rodrigo Santos2, Sara Canas1
1Instituto Nacional de Recursos Biológicos, I.P., INIA - Dois Portos, Quinta dAlmoínha 2565-191, Dois Portos, Portugal.
2JohnsonDiversey Portugal, S.A., Av. Dr Luis Sá, Nº 6, Zona Industrial Abrunheira, Sintra, Portugal
SUMMARY
Winery hygiene is a key factor to wine quality. In this work the effectiveness of four sanitising commercial products over stainless steel tanks was evaluated at a pilot scale. To carry out the experiment a young, untreated red wine, with a very high microbial contamination was distributed by stainless steel tanks where it stood for 70 to 80 days. After that contact time, the tanks were cleaned with an alkaline detergent and sanitised with the four products under study in three concentrations (in triplicate). The monitoring of the process was performed by ATP bioluminescence and traditional plate counts for yeasts, lactic acid bacteria and acetic acid bacteria. Weak correlation was found between the results obtained by the two methodologies. Higher concentrations than the lower recommended for the two oxidising products (peroxyacetic acid and permanganate based) were necessary in order to achieve satisfactory cleanliness. Inferior concentration than the lower recommended was necessary for the products indicated to clean in one step, but it should be noted that in the present work a previous cleaning procedure was conducted.
Keywords:winery, cleanliness, wine microorganisms, ATP bioluminescence, plate counts
Eficácia de Produtos de Higienização Comerciais - Um Ensaio em adega
RESUMO
A higienização da adega é um procedimento chave para a qualidade do vinho. Neste trabalho foi avaliada a eficácia de quatro produtos comerciais na higienização de depósitos de aço inoxidável, em condições de adega. Para a realização do ensaio foi utilizado um vinho tinto jovem não-tratado, com uma carga microbiana elevada, o qual foi distribuído pelos depósitos e neles permaneceu durante 70 a 80 dias. Após esse tempo de contato, os depósitos foram limpos com um detergente alcalino e higienizados com os quatro produtos em estudo em três concentrações (em triplicado). A monitorização do processo foi realizada por análise de bioluminescência-ATP e por métodos tradicionais de contagem por cultura em placa específicos para leveduras, bactérias lácticas e bactérias acéticas. Foi detectada uma fraca correlação entre os resultados obtidos pelas duas metodologias. Para a obtenção de uma limpeza satisfatória com os dois produtos oxidantes (à base de ácido peroxiacético e de permanganato) foi necessária uma concentração superior à mínima recomendada pelo fabricante. Com os dois produtos recomendados para a higienização num só passo (limpeza e desinfecção) a concentração necessária foi inferior à mínima recomendada pelo fabricante, mas deve ser salientado que o procedimento realizado incluiu uma limpeza prévia com detergente alcalino.
Palavras-chave: adega, higienização, microrganismos do vinho, bioluminescência-ATP, cultura em placa
INTRODUCTION
Hygiene is a primordial request in the winemaking industry and its main goal is to remove contamination sources, to control crossed contaminations thus avoiding the dissemination of agents harmful to human health or wine quality. Hygienisation usually consists of two operations, cleaning and sanitation. Cleaning involves the removal of soil and mineral and organic material, which may contain microorganisms or promote microbial growth, from surfaces and equipments by the use of chemical agents and mechanical action. Sanitation refers to the reduction of viable cell populations to acceptably low numbers. Despite cleaning can remove 90% or more of the microorganisms, sanitation is essential to destroy them and avoid their deposition at other locations (Simões 2005; Fugelsang and Edwards, 2007).
Winery residues can be mineral, mainly potassium bitartrate, and organic (originating from grape must, wine and microbial contamination) that may comprise colouring matter, proteins, organic acids, sugars and microorganisms (Fugelsang and Edwards, 2007). These complex biofouling deposits often form more rapidly and are more tightly bound than biofilm alone (Storgards, 2000). Chemical cleaners have been found to be more effective in eliminating attached bacteria from surfaces than disinfectants. Chelating agents such as EDTA (ethylene diamine sodium tetracetate) included in detergent formulations improve the capacity to break biofilms (Storgards, 2000). Various antimicrobial products as well as sanitation formulations are commercially available. The mechanisms of action reflect this diversity, although depending on the microorganism (cell morphology, physiological status) and the events involved, include membrane disruption, macromolecule dysfunction and metabolic inhibition (Denyer and Stuart, 1998). The consequences of the products usage, like the production of off-flavours, material corrosion, low rinsablility, and ecological risk should also be taken into account (Orth, 1998, Storgards, 2000). For example, chlorine based sanitizers have proved to be very effective against wine yeasts but as they are corrosive towards stainless steel and may conduct to the production of off-flavours its use is controversial (Fugelsang and Edwards, 2007; Tristezza et al. 2010).
The effectiveness of sanitation procedures must be evaluated afterwards. In order to implement an integrated cleaning monitoring strategy, visual, non-microbiological and microbiological methods should be combined. An evaluation over time should also be applied and would help recognizing problematic areas. This strategy should ensure that if a surface is unsatisfactory cleaned, the problem can be identified and corrective actions can be carried out (Ogden 1993; Moore and Griffith, 2002a).
The most commonly used methods are swabbing followed by traditional plate counts and ATP bioluminescence using commercial kits. But other methods like agar contact or agar flooding methods, direct epifluorescence microscopy (DEM), protein detection and impedimetry can be used depending on the facilities and the aimed objectives (Storgards, 2000; Moore and Griffith, 2002a; Moore and Griffith, 2002b; Fuster i Vals, 2006).
Swabbing techniques have the advantage to be versatile, followed either by traditional plate counts or ATP bioluminescence, as they can be used in irregular surfaces or confined spaces. Nevertheless, Moore and Griffith (2007) have observed that the swab type as well as the wetting solution can influence the number of bacteria recovered, which is usually low. At a laboratory scale, using a single microorganism, high correlation between ATP bioluminescence and plate counts results, has been found, but, when that comparison is performed at an industrial scale, with the presence of soil and biofilms the correlation found between results has been low (Ogden 1993; Davidson et al., 1999; Storgards, 2000; Moore and Griffith, 2002a; Moore and Griffith, 2002b; Chen and Godwin, 2006; Fuster i Vals, 2006).
The major advantage of ATP bioluminescence test to assess microbial contamination on surfaces is the easiness to perform and quickness of results obtained enabling immediate measures. However, it also detects soil and not just microorganisms (Storgards 2000; Chen and Godwin, 2006; Fuster i Vals, 2006). Another disadvantage of this methodology is that this measurement may be affected by cleaners or sanitizers, and quenching or enhancement effects have been observed, therefore residues of these products may lead to false-negative or false-positive results, respectively (Velazquez and Feirtag, 1997).
Plate counts have the disadvantage to use selective culture medium/conditions of growth and the number of injured cells, viable but not culturable is unknown (Storgards, 2000). Whenever possible it would be advisable to use different methodologies as they reveal different aspects of surface cleanliness.
The aim of the present work was to evaluate the efficiency of four commercial products from Diversey on the sanitation of wine stainless steel tanks at a pilot scale. For each product three in-use concentrations were assayed and cleanliness was evaluated by ATP bioluminescence, and traditional plate counts.
MATERIAL AND METHODS
Wine
A red wine (1500 L) was vinified in 2008 harvest at INIA-Dois Portos winery and left in a stainless-steel tank at open air in order to increase microbial contamination. After 80 days the wine revealed a very high level of microorganisms namely yeasts (including Dekkera/Brettanomyces yeasts), lactic acid and acetic acid bacteria (> 3000 colony forming units or cfu/mL). This wine was equally distributed (40 L) by 36 stainless steel tanks properly cleaned and sanitised (bioluminescence levels below 12 RLU), where stood for 70 days.
Products
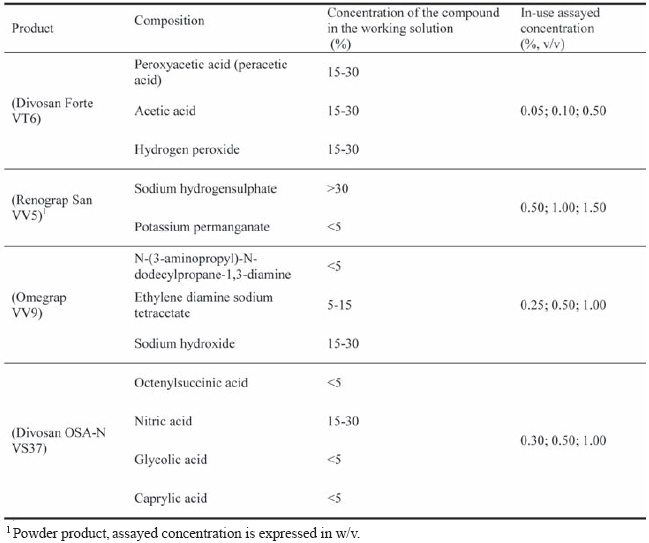
The products composition and in-use assayed concentration are presented in the Table I.
TABLE I
Composition and in-use concentration of the tested products (Diversey).
Composição e concentração utilizada dos produtos testados (Diversey).
Tanks cleaning and sanitising
The tanks mentioned above were emptied, cleaned and disinfected following JohnsonDiversey Portugal instructions.
The cleaning procedure consisted of a rinse with water, followed by cleaning with Spraygrap Liquid VV10 (Diversey; sodium hydroxide, 30 %) in a 2% (v/v) in-use concentration and a volume of 20 mL/dm2 of tank surface. Application was performed with a 5 L sprayer during 5 minutes at winery temperature (approx. 18 ºC).
Tanks were then thoroughly rinsed with water and sanitised with the four products in test. Sanitation consisted of application of 20 mL/dm2 of each product with a 5 L sprayer, at the in-use concentrations indicated at Table 1, in triplicate tanks, during 20 min at winery temperature as represented in Figure 1. A rinse with water finished the process.
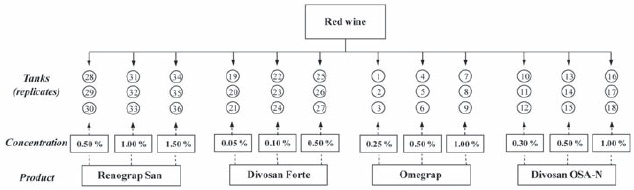
Figure 1 - Schematic representation of the winery assay
Esquema do ensaio realizado em adega
Evaluation of the process
The efficacy of the process was evaluated by ATP-bioluminescence by collecting samples after sanitation at the wall and bottom of all the tanks. Plate counts of yeast, lactic acid and acetic acid bacteria were also used to evaluate the process and sampling took place after detergent cleaning on two randomly chosen tanks, and after sanitation, on at least one of the triplicate tanks.
Additional controls, related with tanks cleaning, were performed for tanks 1 to 18 consisting of: ATP bioluminescence evaluation of all triplicate tanks wall after detergent cleaning; plate counts of one of the triplicate replica tanks before and after detergent cleaning.
ATP bioluminescence assay
The Ultrasnap ATP Swab (Hygiena, USA), which contained a bud pre-moistened with a detergent, was used for surface sampling. An area with 100 cm2 was thoroughly swabbed by zigzagging and rotating the swab. Upon activation, the light emitted in the luciferase mediated reaction was measured and displayed in relative light units (RLU) by a portable SystemSURE II luminometer (Hygiena, USA), according to the manufacturers instructions.
Plate counts assay
For plate counts assay, samples were collected with a cotton swab (Copan Italia, Italy) by swabbing in the way described for bioluminescence: an area of the walls and bottom of the tanks totalizing 100 cm2. The swab was vigorously agitated by vortexing in 5 mL of diluent solution (8,5 g/L sodium chloride, 1,0 g/L de tryptone, sterilized by autoclaving), 1 mL of which was used for plate counts of the following wine associated groups of microorganisms: yeasts, lactic acid bacteria and acetic acid bacteria, using membrane filtration method. The procedures recommended by the Recueil des Méthodes Internationales dAnalyse – Analyse Microbiologique des Vins et de Moûts (OIV, 2008) were followed. The presence of Dekkera/Brettanomyces yeasts was also evaluated by membrane filtration of 1 mL of the above mentioned sample, followed by incubation on DBDM (Dekkera/Brettanomyces differencial medium) culture medium (Stab Vida, Lda., Portugal).
RESULTS AND DISCUSSION
Renograp San
Renograp San is a product based on potassium permanganate which is a strong oxidant, although is not considered to be biocide. Because of its recommendable application in the treatment of wooden barrels, which lacks of suitable products, its efficacy was also tested. The results of the evaluation of the cleanliness of the wine tanks surface after Renograp San usage are presented in Figure 2. Bioluminescence values obtained were generally higher in the walls than in the bottom of the tanks. This difference might be resultant from the strong development of microorganisms that occurred at the surface of the wine and to their adhesion to the tanks wall, which was visible even after wine removal (Figure 3).
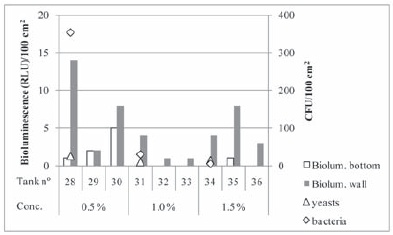
Figure 2 - Bioluminescence and plate count results obtained for each tank after hygienization with Renograp San at the indicated in-use concentrations.
Resultados de bioluminescência e contagens em placa obtidos após higienização com Renograp San, em diferentes concentrações.
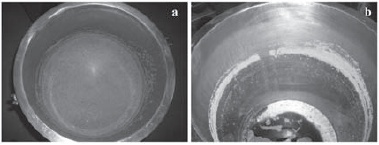
Figure 3 – Wine tank appearance: (a) with contaminated wine; (b) after wine removal.
Aspecto de um depósito: (a) com o vinho contaminado; (b) após a remoção do vinho.
Even though the highest value of bioluminescence corresponded to the highest detection of microorganisms (355 cfu/100 cm2 of bacteria, mainly lactic acid bacteria) the number of results obtained with both methodologies is too small to establish a correlation among them. Though there are no reference values for the wine industry in what concerns the surface cleanliness limit of acceptability, the high bacteria counts (higher than 100 cfu/100 cm2) observed with the lower concentration of product led us to consider the surface cleanliness as unsatisfactory. Moore and Griffith (2002a), based on previous works, have adopted the value of 250 cfu/100 cm2 as a limit for microbial contamination of sanitized food surfaces. Different values have been implemented by other authors for satisfactory-unsatisfactory cleanliness such as 1-10, 300-500 cfu/100 cm2, and values in the middle were pointed out as acceptable (Ogden, 1993; Fuster i Valls, 2006). Regarding bioluminescence, the limit values adopted are also dependent upon the apparatus system, and for example for the Biotrace system, Moore and Griffith (2002a) established 500 RLU as cleanliness limit, and Fuster i Valls (2006) recognized 850 RLU and 1700 RLU/100 cm2 as the lower limit and the upper limit of surface cleanliness acceptability, respectively. In our experimental conditions, according to System Sure II manufacturer, readings less than 10 RLU/100 cm2 indicate that the surface is considered satisfactory cleaned, readings between 11-29 RLU/100 cm2 indicate an acceptable cleanliness and over 30 RLU/100 cm2 indicate that the surface is unsatisfactory cleaned. Analysing the bioluminescence values obtained after Renograp San usage, the surfaces cleanliness can be considered satisfactory as only one value was higher than 10 RLU. For the two higher in-use concentrations of Renograp San assayed in the present work no considerable counts of microorganisms were found (lower than 100 cfu/100 cm2), however the Dekkera/Brettanomyces test gave positive detection for tank 34 (1.50 %). Further analysis should be carried out as Barata and co-authors (2008) have observed that other yeast species can grow on DBDM culture medium and possess some of the Dekkera/Brettanomyces characteristics, unlike the indication given by the culture medium manufacturer. It must be noticed that suspension tests to evaluate fungicidal activity of Renograp San over one strain of Dekkera bruxellensis demonstrate efficacy at 1.00 % in-use concentration (Lonvaud and Gervais, 2003). The results obtained showed that in our experimental conditions Renograp San was efficient at 1.00 % and higher in-use concentration, which is two folds more concentrated than the lower concentration recommended by the manufacturer.
Divosan Forte
The results of the evaluation of sanitation performed with the oxidizing disinfectant Divosan Forte are presented in Figure 4. For all tested product concentrations the bioluminescence values obtained were very low and, moreover higher values were also found for the tanks wall than for the tanks bottom. In this case, there is high correlation among bioluminescence values and yeast counts (r=0.91). In what concerns bacteria high counts were found at the tanks sanitized with the lower concentration of product, with 180 and 250 cfu/100 cm2 (exclusively lactic acid bacteria) detected for two of the three replicates. These values are above 100 cfu/100 cm2 and as was observed for the lowest concentration of Renograp San the cleanliness was not considered acceptable.
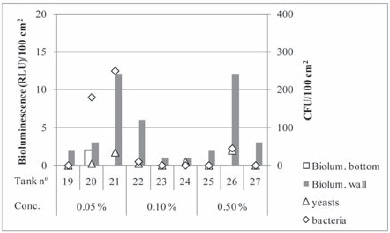
Figure 4 - Bioluminescence and plate count results obtained for each tank after hygienization with Divosan Forte at the indicated in-use concentrations.
Resultados de bioluminescência e contagens em placa obtidos após higienização com Divosan Forte, em diferentes concentrações.
For Divosan Forte at the two higher tested concentrations, zero or low levels of microorganisms were detected, with only one tank (26) presenting 45 and 40 cfu/ 100 cm2 for bacteria and yeasts, respectively. It is noteworthy that in a laboratorial assay performed by Tristezza et al. (2010) a similar oxidising disinfectant, but with three times lower peroxyacetic acid concentration, was effective against suspensions (planktonic state) of wine yeasts and bacteria at in-use concentrations of 0.16 %. However, against the same microorganisms in biofilms higher in-use concentrations were needed and could attain 1.25 % for most of the six yeasts tested. In our experimental conditions we can conclude that Divosan Forte applied in-use concentrations of 0.10 % (v/v) or higher enables a satisfactory level of cleanliness, which is three times higher than the lower concentration recommended by the manufacturer.
Omegrap
Omegrap presents detergent and biocide activity due to its composition: sodium hydroxide, EDTA and an alkylamine. The result of the evaluation of the efficacy of Omegrap at the three tested concentrations is presented in the Figure 5. Variations of bioluminescence values can be noticed, the tanks wall tend to present higher values than the bottom. Nevertheless all bioluminescence values were low, bellow 15 RLU and the majority was even bellow 10 RLU, which corresponds to satisfactory surface cleanliness. Plate counts confirm bioluminescence results as low bacteria (mainly lactic acid bacteria) or yeast cfu were detected. It is worth mentioning that this product was also tested in laboratory by Tristezza et al. (2010) and the minimum inhibitory concentrations or minimum lethal concentrations was 0.16-0.31 % for wine yeasts and an equal value of 0.02 % for wine bacteria, respectively, displaying thus a more pronounced effect over bacteria. However in our experimental conditions a higher number of bacteria cfu was detected for the higher in-use product concentrations. The mentioned authors observed that this product was also effective in removing different yeasts biofilms but the concentrations needed were thirty times higher (5.00 %). It is important to remind that in our experimental conditions this product was used after detergent cleaning and as it was observed by Gronholm et al. (1999) there was a lower efficacy of the alkyl amine based disinfectant when tested on soiled than on previously cleaned surfaces. The values obtained in the present work, both for bioluminescence and plate counts, showed that Omegrap was efficacious at all three concentrations, which corresponds to one third of the lower recommended concentration by the manufacturer.
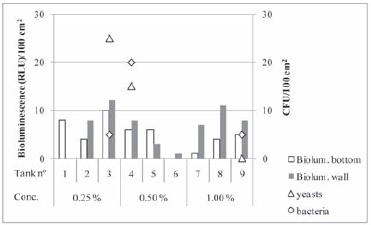
Figure 5 - Bioluminescence and plate count results obtained for each tank after hygienization with Omegrap at the indicated in-use concentrations.
Resultados de bioluminescência e contagens em placa obtidos após higienização com Omegrap, em diferentes concentrações.
Divosan OSA-N
Divosan OSA-N is an acidic detergent and disinfectant for CIP (cleaning in place), recommended for the food and beverage industries for cleaning in one step. It is composed of nitric acid, which is a strong acid presenting cleaning properties, and also weak acids with biocide properties power such as octenylsuccinic acid and caprylic acid (Lopes and Stanton, 1987). The results obtained with Divosan OSA-N are presented in Figure 6. In fact the majority of bioluminescence values were under 10 RLU, indicating satisfactory cleanliness of the surface with all product concentrations. Low plate counts were also found for all assayed concentrations, never exceeding 10 cfu/100 cm2. It is noteworthy that Dekkera/Brettanomyces test was positive for tank 15, however, as mentioned above further tests should have been done in order to confirm the presence of such dangerous spoiler yeasts. Laboratory suspension tests to evaluate fungicidal activity of Divosan OSA-N over one strain of Dekkera bruxellensis revealed efficacy for the lowest in-use concentration tested, 0.50 % (Duarte, 2009). In view of the results obtained for both bioluminescence and plate counts, we can conclude that Divosan OSA-N was effective in all concentrations tested in the present conditions of assay, corresponding to concentrations similar to those recommended by the manufacturer.
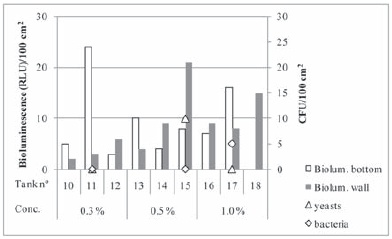
Figure 6 - Bioluminescence and plate count results obtained for each tank after hygienization with Divosan OSA-N at the indicated in-use concentrations.
Resultados de bioluminescência e contagens em placa obtidos após higienização com Divosan OSA-N, em diferentes concentrações.
Detergent cleaning procedure evaluation
The detergent cleaning procedure was initially planned to be evaluated for two tanks randomly chosen, as all the nine tanks per product were subjected to similar procedures. Surfaces were sampled before disinfection and plate counts of the groups of tested microorganisms were performed. No microorganisms were detected for any of the surfaces sampled (tanks 28, 31, 19 and 26). This was a rather puzzling result as microorganisms were detected after disinfection. In this way we decided to perform a more accurate control of the tanks surface before cleaning and disinfection.
The results obtained for tanks 1 to 18 before and after cleaning with detergent Spraygrap are separately presented in Figures 7 and 8, corresponding to different days of experiment (products Omegrap and Divosan OSA-N). Despite the analogous treatment applied to every tank presented at Figures 7 and 8, differences between bioluminescence as well as plate count values among tanks, were observed, and were more acute before cleaning with the detergent. Several aspects might contribute to that variation. One could be related to the high heterogeneity associated with surface contamination or sanitation level. In fact, Fuster i Valls (2006), using direct epifluorescence microscopy (DEM), verified that different microscopic fields of stainless steel discs, with an area as small as 3 cm2, were quite different in terms of sanitation. For each disc, fields without microorganisms and soil, fields with soil and fields with both soil and microorganisms were observed. Another source of variation may be the presence of biofilms, as microorganisms within a biofilm are not uniformly distributed. In a biofilm attached cells divide forming microcolonies and produce extracellular polymeric substances forming a matrix that stabilises the colonies (Storgards, 2000).

Figure 7 - Bioluminescence (wall and bottom) and plate count results obtained for the tank series 1-9 before and after detergent cleaning.
Resultados de bioluminescência (parede e fundo) e contagens em placa obtidos para a série de depósitos 1-9 antes e após a limpeza com o detergente
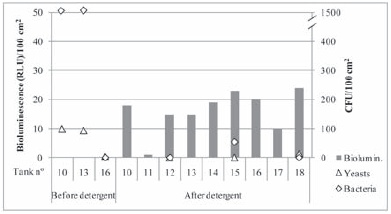
Figure 8 - Bioluminescence (wall and bottom) and plate count results obtained for the tank series 10-18 before and after detergent cleaning.
Resultados de bioluminescência (parede e fundo) e contagens em placa obtidos para a série de depósitos 10-18 antes e após a limpeza com o detergente.
On the other hand, sampling techniques can also contribute to that variation due to the fact that it is extremely difficult to remove attached cells quantitatively (Storgards, 2000). Swabbing techniques, have been extensively studied, namely with bioluminescence, using commercial kits, and traditional hygiene swabbing (followed by plate counts) using different types of swabs and removing solutions andgenerally results showed low recovery rates of inoculated bacteria and low reproducibility (Davidson et al., 1999; Moore and Griffith, 2002b; Moore and Griffith, 2007).
Observing Figures 7 and 8 high counts of microorganisms were detected before cleaning, mainly lactic and acetic acid bacteria. After cleaning low numbers of microorganisms were detected as well as low values of bioluminescence (wall surface). Only three tanks presented values of bioluminescence higher than 29 RLU (can be considered dirty), 12 tanks between 11 and 29 RLU and 3 tanks presented values lower than 11 RLU/100 cm2. These results show that after detergent cleaning the surface seems to present a satisfactory cleanliness either in terms of plate counts or bioluminescence. Tristezza et al. (2010) have verified that an alkaline powder detergent based on sodium hydroxide (>30 %) presented minimum inhibitory concentrations (MIC) varying from 0.04 to 0.63 % (in-use concentration) and minimum lethal concentrations (MLC) varying from 0.04 to 0.31 % (in-use concentration) over wine strains of six different yeasts species, one lactic acid and one acetic acid bacteria. This product was also effective in removing biofilms of different wine related yeast species and Acetobacter aceti with concentrations differing from 1.25 % to 5.0 %. In our experimental conditions of assay, 2 % in-use concentration of Spraygrap, there may be some microorganisms still attached to the surface but that could not be removed with the traditional swabbing and therefore low or no microorganisms were detected by plate culture. This hypothesis is also supported by the work of Simões (2005) who has observed that a Pseudomonas fluorescens biofilm was more efficiently removed with a sodium hydroxide solution three times higher than we have assayed, however total biofilm eradication was not achieved. Additionally, at lower concentrations the sanitizers Renograp San and Divosan Forte might have weakened the biofilm (easier removal by traditional swabbing) but a high number of bacteria were still viable and cultivable. To notice that bioluminescence, which swab was impregnated with a detergent solution, though with low levels after detergent cleaning was higher than after sanitation.
Taking into account all bioluminescence values and respective plate counts obtained, low correlation was found among them (yeast r=0.64; bacteria r=0.35). Various works have been conducted in order to estimate relations concerning these two types of analysis and the results have been quite variable, from good correlations to weak correlations (Ogden 1993; Storgards, 2000; Moore and Griffith, 2002a; Moore and Griffith, 2002b; Chen and Godwin, 2006; Fuster i Vals, 2006). Nevertheless, at the present work we have evaluated groups of microorganisms more often related with wine spoilage that are recommended by OIV (2008) to be controlled, and it might be possible that other microorganisms were present and contributed for higher bioluminescence values.
CONCLUSIONS
High heterogeneity was observed for surface contamination and/or cleanliness, evaluated either by ATP-bioluminescence or by plate counts. Low correlation was also found among results obtained from both methodologies. With these experimental conditions, higher concentration of Renograp San (permanganate based) and Divosan Forte (peroxyacetic acid based) than the lower recommended by the manufacturer was necessary in order to obtain satisfactory cleanliness. On the contrary, for Omegrap (alkylamine based) and Divosan OSA-N (octenil-succinic acid based) concentrations bellow the lower recommended by the manufacturer were needed to achieve satisfactory cleanliness. However it is important to notice that these products are formulations with detergent and disinfection activity, to be used in one-step, and in the present experiment a previous cleaning step with an alkaline detergent was performed.
This assay enabled to verify that the cleaning followed by sanitising with adequate sanitizers concentration was effective for the cleanliness of highly contaminated winery tanks.
REFERENCES
Barata A., Seborro F., Belloch C., Malfeito-Ferreira M., Loureiro V., 2008. Ascomycetous yeast species recovered from grapes damaged by honeydew and sour rot. J. Appl. Microbiol., 104, 1182–1191. [ Links ]
Chen F.-C., Godwin S.L., 2006. Comparision of a rapid ATP bioluminescence assay and standard plate count. methods for assessing microbial contamination of consumers refrigerators. J. Food Prot., 69, 2534-2538. [ Links ]
Davidson C.A., Griffith C.J., Peters A.C., Fielding L.M., 1999. Evaluation of two methods for monitoring surface cleanliness - ATP bioluminescence and traditional hygiene swabbing. Luminescence, 14, 33–38. [ Links ]
Denyra S.P., Stewart G.S.A.B. 1998. Mechanisms of action of disinfectants. Int. Biodet. & Biodeg., 41, 261-268. [ Links ]
Duarte, F.L. (2009) Relatório de avaliação de actividade fungicida e bactericida de Divosan Forte e Divosan OSA-N. 6 p., INIA-Dois Portos. [ Links ]
Fugelsang K.C., Edwards C.G., 2007. Winery Cleaning and Sanitizing. In: Wine Microbiology. Practical Applications and Procedures, 139-151. 2nd ed. Springer, New York. [ Links ]
Fuster i Valls N., 2006. Importancia del control higiénico de las superficies alimentarias mediante técnicas rápidas y tradicionales para evitar y/o minimizar las contaminaciones cruzadas. 160 p. PhD thesis, Universitat Autònoma de Barcelona, Bellaterra. [ Links ]
Grönholm L., Wirtanen G., Ahlgren K., Nordström K., Sjöberg A.-M., 1999. Screening of antimicrobial activities of disinfectants and cleaning agents against foodborne spoilage microbes. Z. Lebensm. Unters. Forsch. A, 208, 28/9-298. [ Links ]
Lonvaud A., Gervais J.-P., 2003. Évaluation des activités bactéricides et fongicides de différents produits de désinfection Johnson Diversey. 14 p. Rapport dexpérimentation, Service Vignes et Vins/Faculté dnologie, Bordeaux. [ Links ]
Lopes, J. A., Stanton, J. H., 1987.Antimicrobial Sanitizing Composition Containing N-Alkyl and N-Alkenyl Succinic Acid and Methods for Use, United States Patent number 4715980. [ Links ]
Moore G., Griffith C., 2002a. A comparison of traditional and recently developed methods for monitoring surface hygiene within the food industry: an industry trial. Int. J. Environ. Health Res., 12, 317–329. [ Links ]
Moore G., Griffith C., 2002b. A comparison of surface sampling methods for detecting coliforms on food contact surfaces. Food Microbiol., 19, 65-73. [ Links ]
Moore G., Griffith C., 2007. Problems associated with traditional hygiene swabbing: the need for in-house standardization. J. Appl. Microbiol., 103, 1090–1103. [ Links ]
OIV, 2008. Microbiological Analysis of Wines and Musts. Detection, Differentiation and Counting of Micro-organisms. In: Compendium of International Methods of Analysis of Wine and Musts. Vol.2, 21p International Organization of Vine and Wine, Paris. [ Links ]
Ogden K., 1993. Practical experiences of hygiene control using ATP-Bioluminescence. J. Inst. Brew., 99, 389-393. [ Links ]
Orth R., 1998. The importance of disinfection for the hygiene in the dairy and beverage production. Int. Biodet. & Biodeg. 41, 201-208. [ Links ]
Simões M., 2005. Use of biocides and surfactants to control Pseudomonas fluorescens biofilms. Role of the hydrodynamic conditions. 223 p. PhD Thesis. University of Minho, Braga. [ Links ]
Storgards E., 2000. Process hygiene control in beer production and dispensing. 105 p PhD thesis, VTT Technical Research Centre of Finland, Espoo. [ Links ]
Tristezza M., Lourenço A., Barata A., Brito L., Malfeito-Ferreira M., Loureiro V., 2010. Susceptibility of wine spoilage yeasts and bactéria in the planktonic state and in biofilms to disinfectants. Ann. Microbiol., 60, 549–556. [ Links ]
Velazquez M., and Feirtag J.M., 1997.Quenching and enhancement effects of ATP extractants, cleansers, and sanitizers on the detection of the ATP bioluminescence signal. J. Food Prot., 60, 799-803. [ Links ]
ACKNOWLEDGMENTS
We are grateful to Prof. Maria João Sousa, from Minho University, for the fruitful discussion of some results obtained.
(Manuscrito recebido em 11.03.11. Aceite para publicação em 26.05.11.)
*Corresponding author: e-mail: filomena.duarte@inrb.pt













