Introduction
Ovarian torsion is an uncommon cause of acute pelvic and abdominal pain, with an overall estimated incidence of 2-3% of gynaecologic emergencies1. It results from twisting of the ovarian vascular pedicle, leading to partial or complete blood flow obstruction with venous, arterial, and lymphatic stasis. The Fallopian tube often twists along with the ovary, in which cases it is referred as adnexal torsion2,3 (Fig. 1). It is a surgical emergency since treatment delay leads to haemorrhagic infarction, which may ultimately evolve to ovarian necrosis with subsequence autoamputation or superinfection4,5.
While early diagnosis is decisive on the clinical outcome and the possibility of ovarian salvage, it is often challenging due to the nonspecific and/or intermittent nature of the symptoms (episodes of spontaneous torsion and detorsion). These include acute onset of severe abdominopelvic pain, nausea, and vomiting. Laboratory abnormalities are usually absent, however, in longstanding ovarian torsion, ovarian necrosis will elicit a systemic inflammatory response, with fever, leukocytosis, and elevation of nonspecific inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin4.
The most common predisposing factor is an underlying ovarian mass, usually greater than 5 cm, that weights the ovary down and induces it to rotate on the axis of the infundibulopelvic and utero-ovarian ligaments (Fig. 1).
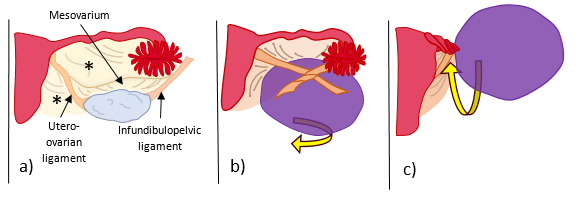
Figure 1: Schematic illustration of a) normal anatomy of the ovarian ligaments: infundibulopelvic ligament (or suspensory ligament of the ovary), which is a fold of the broad ligament (*) that contains the ovarian vessels and is attached to the pelvic sidewall, the utero-ovarian ligament (or proper ovarian ligament) which attaches the ovary to the lateral wall of the uterus, and the mesovarium which attaches the ovary to the broad ligament (*); b) ovarian torsion with twisting around the infundibulopelvic and utero-ovarian ligaments, and c) adnexal torsion with twisting of the Fallopian tube along with the ovarian ligaments.
Radiologic evaluation with pelvic ultrasound (US) as a first-line examination, with or without additional cross-sectional imaging (CT or MRI), is almost always necessary for its diagnosis and to exclude other more common causes of pelvic and lower abdominal pain, such as acute appendicitis, acute diverticulitis, ectopic pregnancy, ruptured ovarian cyst, tubo-ovarian abscess, among others6. The radiological appearance of an ovarian torsion consists of an adnexal mass or an enlarged ovary, with twisting of its vascular pedicle (whirlpool sign), free fluid in the pelvis, uterine deviation towards the side of torsion, and decreased adnexal vascularization.
Case Presentation
A 49-year-old woman presented to the emergency department with acute lower abdominal and pelvic pain, nausea and vomiting. Laboratory tests were unremarkable apart from a slight increase in the creatinine levels, and a CT scan was performed (Fig. 2), showing a large cystic left adnexal mass with 16 x 12 cm, causing extrinsic compression of the left ureter with mild ureterohydronephrosis, and a small amount of ascites, with no further alterations. The patient was discharged with the presumptive diagnosis of an ovarian tumour suspicious for malignancy with probable peritoneal carcinomatosis and was therefore referred to our oncology centre.
A week later, a pelvic MRI was performed in our Radiology department, with the purpose of ovarian characterization. At this point, the patient maintained abdominopelvic pain and nausea (assumed to be caused by tumour compression of the gastrointestinal tract) and had developed fever (38,3ºC). Laboratory tests showed increased CA-125 of 354.9 U/mL (normal levels < 35 U/mL), leucocytosis of 20.300 x 103/uL (normal range: 4.000-10.000 x 103/uL) with neutrophilia, and thrombocytosis of 743 x 103/uL (normal range: 150-400 x 103/uL). C-reactive protein was not requested.
Pelvic MRI showed a large multicystic pelvic tumour arising from the left adnexa, with T1WI hyperintense content that did not enhance in post-contrast sequences, in keeping with subacute intra-tumoural haemorrhage and/or necrosis (Fig. 3).
Although there were no categorical signs of ovarian torsion such as the whirlpool sign, the clinical context indicating systemic inflammation/infection (leucocytosis with reactive thrombocytosis) and the ipsilateral uterus deviation (which would not be expected by the mass effect of a large tumour), the hypothesis of an ovarian tumour with torsion and ovarian necrosis with possible super-infection was raised, and the patient underwent emergent surgical intervention.
At surgery, a left adnexal tumour with torsion was confirmed (3 loops), and adnexectomy was performed. Moderate ascites was present, with no signs of peritoneal metastases. Extrinsic tumour compression of small bowel loops and both ureters was noted, causing partial intestinal and ureteral obstruction, respectively. Uterus and right adnexa were unremarkable.
Histological examination of the surgical specimen revealed a multicystic ovarian tumour with thin walls and septa, measuring 15 x 3 x 8 cm, with extensive necrosis, oedema, and haemorrhagic content, with no signs of malignancy. There was no mucin content. It was not possible to assess the epithelial lining of the cystic cavities due to the extensive necrosis. The cytological examination of the ascitic fluid was negative for neoplastic cells.
A final diagnosis of a benign cystic ovarian tumour with torsion and necrosis was made.
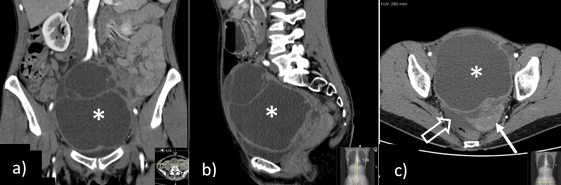
Figure 2: Coronal (a), sagittal (b) and axial (c) contrast-enhanced abdominopelvic CT shows a pelvic midline multiloculated cystic tumour, with thickened wall and non-enhancing content, thought to arise from the left adnexa (a right normal ovary was identified - not shown). Also noted, ipsilateral (left) deviation of the uterus (→) and free intra-peritoneal pelvic fluid (open arrow).
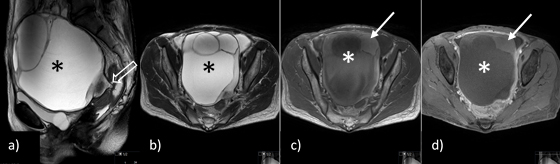
Figure 3: Pelvic MRI: a) sagittal and b) axial T2-weighted imaging (WI), c) axial T1WI and d) fat suppressed T1WI after the administration of contrast, show a large multicystic pelvic tumour (*) arising from the left adnexa, with a beak-like projection to the adnexal region that could suggest a torsed ending (open arrow), although twisting of the ovarian pedicle was not identified, with T1WI hyperintense content that does not enhance in post-contrast sequences in keeping with subacute intra-tumoural haemorrhage and/or necrosis.
Discussion
In ovarian torsion, arterial blood supply impairment is usually preceded by venous drainage obstruction since the thin walls of the veins are more compressible than the muscular walls of the arteries and will collapse first. Continuous arterial perfusion in the setting of a blocked outflow venous system results in ovarian oedema with marked ovarian enlargement. Ischemia then occurs, and if left untreated, it may lead to ovarian necrosis with or without further superinfection.1,3,4
Ovarian torsion may occur in females of all ages, even in foetuses and neonates, and the main risk factors are the presence of an ovarian mass and a history of previous ovarian torsion. Although torsion may occur in normal ovaries, in the majority of cases there is a (usually benign) large (> 5 cm) mass present, more frequently a physiologic cyst (follicle, corpus luteum) or a benign neoplasm. In other cases, an underlying cause for ovarian enlargement such as in ovarian hyperstimulation syndrome or polycystic ovary syndrome is present. Endometriomas and malignant tumours more rarely cause ovarian torsion as they are less mobile and often have adhesions with the surrounding structures.3,4
The most common differential diagnosis that may also present with acute abdominopelvic pain and an adnexal mass includes ectopic pregnancy (which can be excluded by a negative serum human gonadotropin blood test), a ruptured ovarian cyst (which is usually a midcycle occurrence with distinct radiological features that include the presence of a haemorrhagic cyst and haemoperitoneum), and a tubo-ovarian abscess (which has a more indolent course, more often associated with fever and leukocytosis, and is usually depicted by a complex multilocular mass). Isolated fallopian tube torsion may also occur, although this is an extremely rare event, where the ovary remains normal, and tubal dilation with haemorrhage and wall thickening is seen. Similar to ovarian torsion, isolated tubal torsion requires surgical intervention. Infarction of a subserosal uterine or broad ligament leiomyoma may mimic ovarian torsion clinically, and in this scenario identification of an ipsilateral normal ovary is a key finding.1,3
Another important differential diagnosis that should be borne in mind is the sole presence of a cystic ovarian tumour (without torsion), a misdiagnosis that may be avoided when considering the clinical and laboratory background along with the radiological findings. This is well illustrated in our case, which was initially misled by the interpretation of the imaging findings alone, neglecting the clinical context (abdominopelvic pain, vomiting, and signs of systemic inflammation/infection).
While US is the first-line examination for a suspected ovarian torsion, it may not be the first imaging modality performed if other pathologies lead the differential diagnosis, and as such, it is not uncommon to first depict an ovarian torsion at CT or MRI. For this reason, it is very important that the radiologist becomes familiar with the expected imaging features in each modality.
Grey-scale ultrasound findings include an adnexal mass or enlarged ovary (greater diameter > 4 cm or volume > 20 cc) with heterogeneous central follicular stroma (that results from oedema and haemorrhage) and peripherally displaced uniform (8- 12 mm) follicles. Colour and spectral Doppler are helpful in assessing ovarian vascularization, which should be decreased or absent, although it may be normal depending on the degree of obstruction and the chronicity of the torsion. Colour Doppler may also help to depict the whirlpool sign - that is, the characteristic swirling target appearance of the vessels in the twisted pedicle and is considered pathognomonic. Other findings include free pelvic fluid and uterine deviation towards the enlarged ovary.1,3,4,7
The same findings are seen on cross-sectional imaging with CT or MRI. Additional features include subacute haemorrhage (that may be noted as intra-ovarian haematoma, haematosalpinx and/or haemoperitoneum) and is highly associated with infarction and secondary necrosis of the affected ovary. It is best seen on MRI T1-weighted imaging (hyperintense content), or even on unenhanced CT (hyperdense content), with or without a layering hematocrit level. Contrast-enhanced acquisitions will better depict reduced ovary enhancement and vessel engorgement on the twisted side, and often aid in the detection of an underlying mass. Multiplanar acquisitions/reformations are optimal for the detection of the twisted vascular pedicle, ipsilateral uterine deviation, and fallopian tube thickening.1,3,4,5,7
Conclusion
Radiologists play a critical role in the diagnosis of ovarian torsion. However, clinical presentation and imaging findings may both be unspecific, in which case, the correct diagnosis may only arise from a high level of clinical suspicion and comprehension of the pathophysiology that underlies the different chronological stages of ovarian torsion.















