Introduction
The most abundant resource for existing bio-treatment facilities is Bm from Lcs, which comes from softwood, agricultural and ranger service wastes. Recently, there has been a rise in Lcs reductive reactant fractionation cycles, where Ln is first converted into a sweet-smelling feedstock 1-2. Ln extraction is a critical step in the fractionation of raw Bm from Lcs, as part of the pre-treatment process for energy production. Bm from Lcs is composed of three main components: Cs, Hcs and Ln. These components are tightly bound together, and need to be separated to efficiently convert Bm into biofuels and other valuable products 3. Ln is a complex and rigid polymer that provides structural support to plants. However, it poses a challenge during Bm processing, since it can hinder the accessibility of enzymes or chemicals to Cs and Hcs, making their conversion into biofuels or chemicals less efficient 4. Therefore, Ln extraction is required to improve Bm fractionation, and enhance the overall efficiency of energy production. After Ln extraction, the remaining Cs and Hcs-rich fraction becomes more accessible for further processing 5. This can include enzymatic hydrolysis, to convert Cs into sugars, which can then be fermented into biofuels like ethanol, or used as feedstock for other bio-based products. It is important to note that Ln itself is a valuable resource which can be used for various purposes, such as the production of chemicals and materials, and even as a source of renewable energy, through processes like combustion or gasification 6. Overall, Ln extraction plays a crucial role in the efficient utilization of Bm from Lcs, for producing energy and developing a sustainable bio-based economy. The choice of Ln extraction methods depends on factors such as Bm type, end product goals and economic considerations 7. Researchers and industries have been working on developing innovative processes and technologies to convert Bm from Lc into valuable chemicals. These efforts include Bm pretreatment, enzymatic hydrolysis, fermentation and advanced separation and purification techniques 8. However, it is important to acknowledge that the use of Bm from Lc for chemicals is still an ongoing area of research and development. Challenges such as efficient Bm deconstruction, cost-effective processing and economically viable pathways development for chemical production remain to be addressed 9. Nonetheless, the increasing emphasis on sustainable and environmentally friendly practices in chemical industry has spurred significant interest and investment in leveraging Lc feedstocks as a key component of the transition towards a more sustainable and circular economy 10. Ln extraction is a crucial step in processing Bm from Lc for various applications, including energy production and chemical manufacturing. Ln can be isolated from different Bm sources, such as wood, agricultural residues (wheat straw and rice husk) and industrial byproducts (sugar cane bagasse), through various extraction processes 11. These processes aim to separate Ln from other Bm components, particularly Cs and Hcs, to enable their utilization 12. Research on Ln chemistry dates back to the mid-nineteenth century, and it has played a significant role in the development of new materials and use of Bm from Lc as a renewable resource. The understanding of Ln properties and structure has paved the way for various applications. Advancements in technologies related to papermaking, wood processing and textiles have further contributed to the development of Ln-based polymers and the establishment of biorefinery concepts 13. Ln integration into biorefinery systems allows for a more comprehensive and efficient utilization of Bm from Lc. Ln can be converted into high-value chemicals, like aromatic compounds and bio-based polymers, as well as used for energy generation through combustion or gasification. In recent years, ongoing research has further advanced our understanding of Ln complex chemistry, and its potential applications 14. This research has led to the development of new methods for Ln extraction, modification and use, contributing to economic growth and a transition towards more sustainable and renewable resources 15. This research paper aims to provide background information on the economic aspects related to Ln use, including the entire value chain, from its extraction, using various processes, to its subsequent thermal conversion or use for biofuel production. It goes beyond presenting the various chemical investigations conducted on Ln, focusing on highlighting the potential applications for chemicals and polymers production 16. By providing a comprehensive overview of the economic aspects and potential applications of Ln, this review can contribute to a better understanding of its role in a sustainable bioeconomy. It can also inform policymakers, researchers and industry stakeholders about the opportunities and challenges associated with employing Ln as a valuable and versatile feedstock for chemicals, polymers and renewable energy 17.
Materials and methods
Materials selection
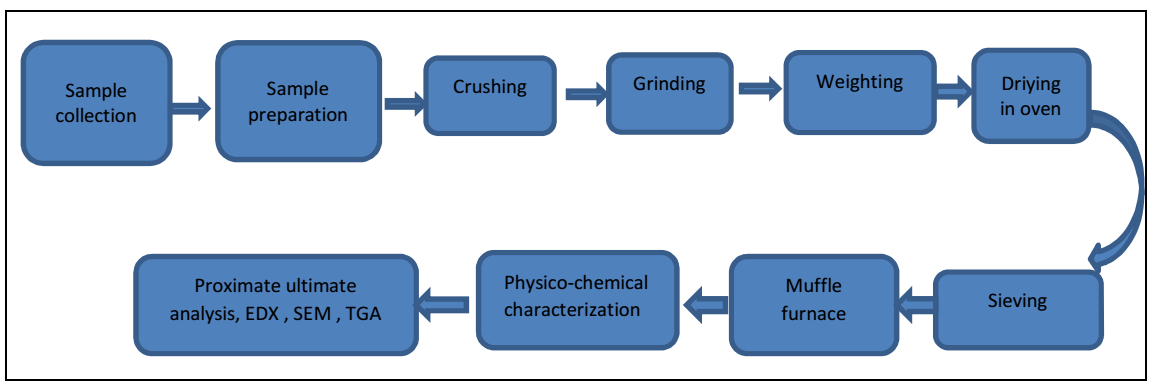
Four Bm sources are presented in this research paper. BTB and NTB were collected from different areas of Jamshoro. AS and WS were purchased from Quetta Kandahar bazaar shops, Baluchistan, Pakistan. Fig. 1 shows their experimental research methodology.
LN extraction from ANs, BTB, NTB and WNs
Ln extraction from and AS, BTB, NTB and WS (Fig. 2) was carried out by the following method. 2 wt% NaOH, i.e., 20 g NaOH + 1000 mL distilled water, were mixed. Then, the solution was heated in an oven, at a T of 100 ºC, for 5 h. Afterwards, the liquor was mixed with Bm, in the ratio of 1:20. 50 g Bm and 1000 mL liquor were kept in the oven, for different times and T. Ln from liquor was used as extracted by filtration with an agitated magnetic heater, and washed with distilled water, to remove excess alkali. The aqueous filtrate was acidified to pH 1, with concentrated H₂SO₄. A pipette was used for taking dropwise H₂SO₄, and a pH meter was employed. Then, the mixture was allowed to boil for 1 h. Afterwards, it was cooled down, and distilled water was added to the solid residue. The precipitate obtained in this operation was separated by a filter paper, washed with distilled water, until pH 7 was achieved. Unwanted solids and impurities were removed. The resulting Ln samples were dried in the oven, at 110 ºC, for further treatment.
Results and discussion
Proximate and ultimate analyses
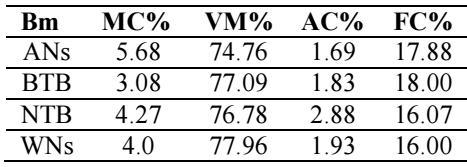
Proximate analyses of MC, VM, FC and AC was carried out for 4 h, at 650 ºC, in an electric muffle furnace. Table 1 and Fig. 3 show Bm results for these parameters.
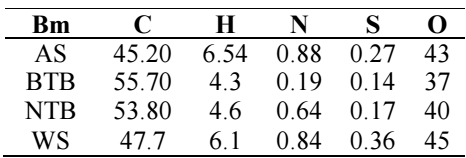
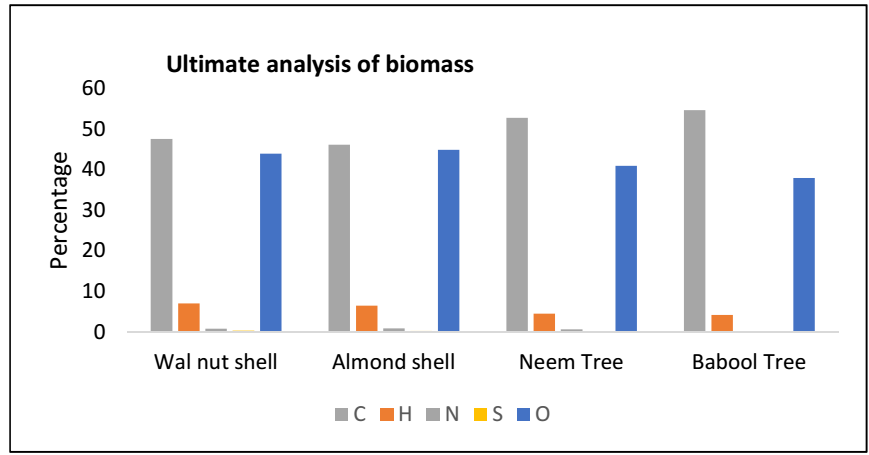
With a sample mass of 350 mg, O gas and a SC 832 LECO model, the final analysis of the raw sample was performed. The material was sieved at 150 µ, and dried at 105 °C, for 1 h, to get the results on a dry basis. Table 2 and Fig. 4 show the results of the raw sample final analyses, including C, H, N and O.
The raw material for BTB analysis had the highest Ct of C and the lowest S content. AS content of O was higher than that of BTB. While reducing N and S levels, acid washing may slightly increase Ct of C, H and O in Bm from Ln.
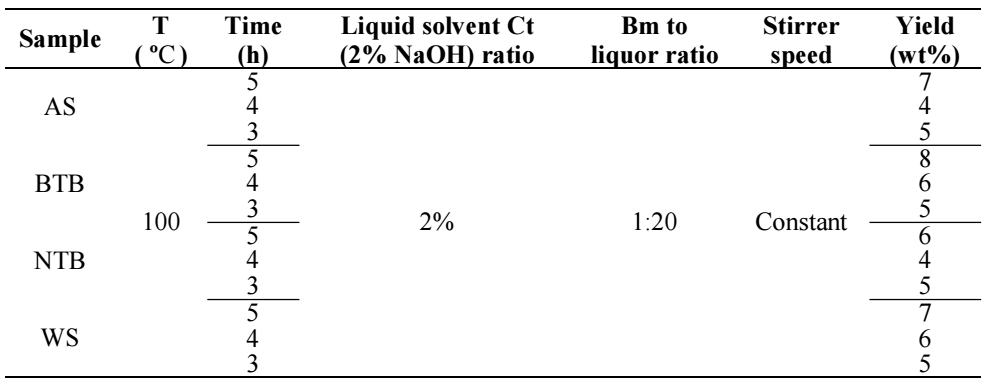
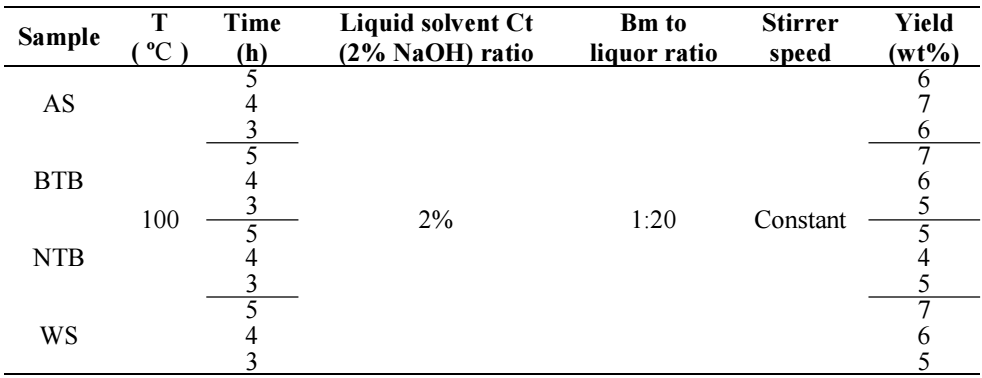
Tables 3 and 4 present results on Ln yield extraction for the different samples tests. For all four Bm samples, the time was 5, 4 and 3 h. T values were 100 and 160 ºC. Both Bm-to-liquor ratio and the liquid solvent Ct were held constant at 2% and 1:20, respectively. To investigate T effect on Ln extraction yield from Bm, experiments, showing the different liquor formulation used in the complete methodology of this work on LN extraction were conducted.
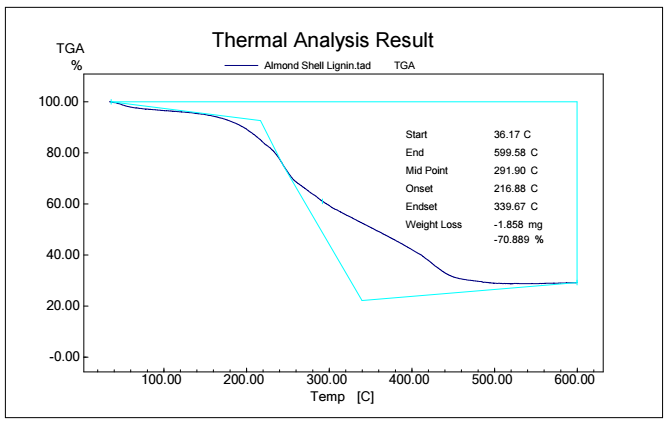
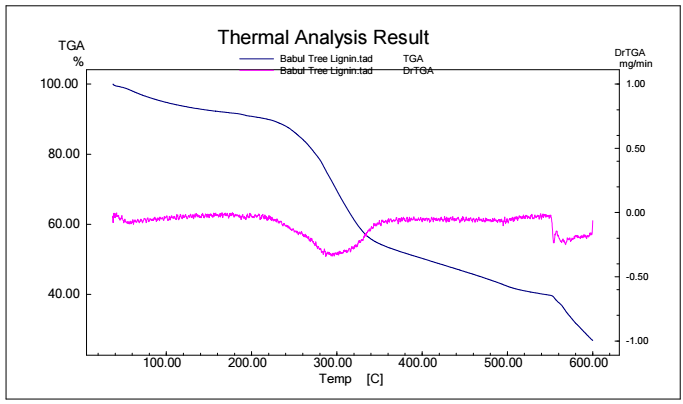
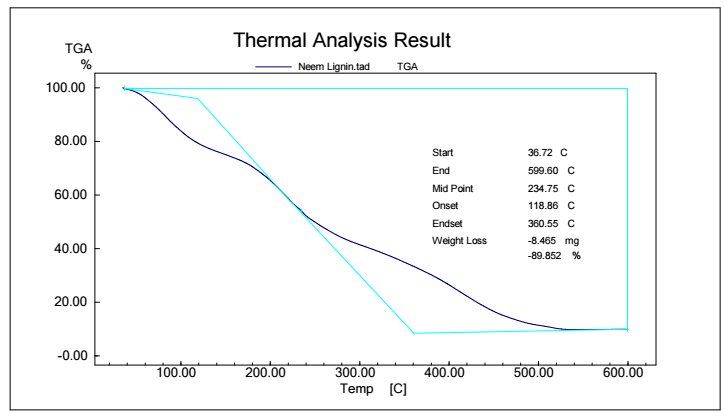
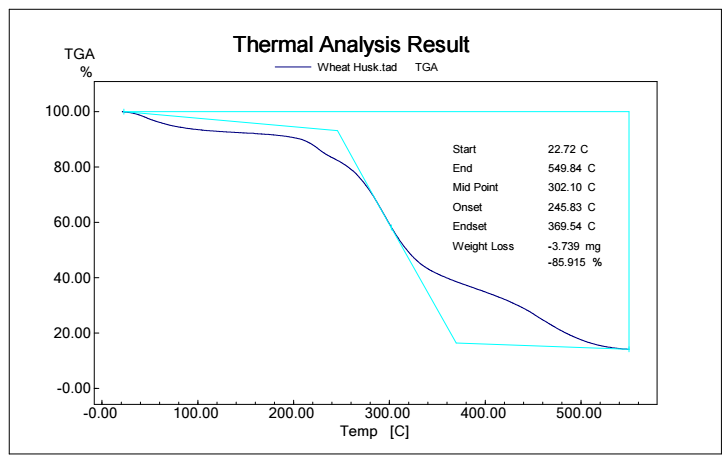
Figs. 5, 6, 7 and 8 show TGA analysis of Bm from AS, BTB, NTB and WS extracted Ln. As observed in Figs. 5 and 6, if the percentage change in sample mass decreased with an increase in T in a TGA curve plot, which typically indicates a process of sample mass loss or decomposition.
The derivative TGA curve, shown in Fig. 6, is most likely what the name "DrTGA" in TGA analysis refers to. DrTGA, which is created from TGA data, discloses the rate of weight loss or gain, as a function of T or time. DrTGA of AS is almost -0.25 mg/min, at a nearly constant (60%) TGA percentage and varied T (Fig. 7). A negative DrTGA indicates weight gain due to T or time, as opposed to weight loss. This shows that, while undergoing thermal analysis, the WS sample gained weight. A negative DrTGA curve may indicate a variety of environmental variables. It might also mean that a chemical reaction took place in the sample, causing a net mass gain. It is essential to analyze TGA data, along with other complementary methods, to completely understand the sample thermal behavior. To assess the accompanying heat movement during thermal events, TGA is usually paired with DSC, which provides further insights into the sample behavior during T changes. A 10% weight loss in TGA (as depicted in Fig. 7) signifies that 10% Ln from NTB initial mass was lost due to thermal changes such as breakdown, evaporation or other processes. The derivative TGA curve for BTB is shown in Fig. 8.
Conclusion
One potential source for sustainable raw materials is lignin. The present investigation describes Ln extraction from Bm of AS, BTB, NTB and WS resources. Additionally, Ln extracted from Bm of BTB had positive yields compared to that from NTB. It was concluded that Lc yield was influenced by a change in time and T. After 5 h, and at 100 ºC, BTB achieved Ln yield of 8%, while NTB attained 6%. However, Ln recovered from WS and AS was 7%, at a T of 160 ºC, after 5 h. Finally, it was decided to employ acid and alkali for the process. The extracted material was characterized by TGA. Thus, this biodegradable and biocompatible Ln recovered from Bm, by alkaline pretreatment, is more valuable for commercial and domestic use, as a polymeric material The alkaline treatment was able to remove Ln from Bm of AS, BTB, NTB and WS, according to instrumental analyses findings. However, a small portion of sugar impurities remained in Bm-derived Ln, which can be removed by acid treatment. Lignin gets mostly converted and degraded in laboratories.
Acknowledgment
The staff in the Department of Chemical Engineering and Technology at MUET in Jamshoro, Pakistan, is sincerely appreciated by the authors, for their significant guidance and assistance in carrying out the current research work.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
AC: ash content
AS: almond shell
Bm: biomass
BTB: Babul tree bark
Cs: cellulose
Ct: concentration
DSC: differential scanning calorimetry
FC: fixed carbon
H2SO4: sulfuric acid
HCs: hemicellulose
LCs: lignocellulose/lignocellulosic
Ln: lignin
MC: moisture content
NTB: Neem tree bark
T: temperature
TGA: thermogravimetric analysis
W%: weight percent
WnS: walnut shell
VM: volatile matter